Introduction
According to WHO, Pharmacovigilance is “The science and activities concerned with detection, assessment, understanding, and prevention of adverse drug reactions and drug-related problems. Its role in ensuring public health and medication safety cannot be overstated.”
Objectives of pharmacovigilance
Improve patient care and safety associated with the use of medicine.
Improve public health and safety related to the use of medicine.
Evaluate the advantages, efficacy, and risks of medication.
Encourage education, understanding, and clinical training in Pharmacovigilance.1
Drugs
A drug is any substance other than food used in the prevention, diagnosis, aviation, or treatment of a disease that is called a drug. Drugs are available as legal or illegal drugs.
Illegal drugs
The Food and Drug Administration (FDA) has studied these chemical substances and found that they cause more harm than good. A person caught posing, selling, or using can be arrested, fined, and sent to jail.
Examples: Cocaine, Heroin, Illegal steroids.
Legal drugs
Those drugs that have been studied, texted, approved, and controlled by the FDA, such as prescription and OTC drugs.
Prescription drugs
These are prescribed by a legal Medical Practitioner who tells the pharmacist to dispense a particular medication. These are prescribed to treat a specific medical problem.2
OTC drugs
These drugs are easily accessible from pharmacies without a prescription from a health professional. They are safe for short-term use and effective for minor common diseases. The advice of a pharmacist is sufficient as directed on the label. The Food and Drug Administration (FDA) in the USA defines OTC drugs as safe and effective when used according to the label without a prescription or according to a health professional’s advice, if necessary. Self-medication is medication taken by patients responsible for treating their minor illnesses.
Classification of OTC drugs
The most used over-the-counter medications include:
Pain relievers - Acetaminophen, Aspirin, Ibuprofen, and Naproxen.
Medicines for heartburn and indigestion - Omeprazole, Lansoprazole, Cimetidine, and Aluminum Hydroxide.
Laxatives - Bisacodyl and Calcium Docusate.
Antidiarrheal drugs - Bismuth Sub Salicylates and Loperamide.
Motion sickness medicines - Dimenhydrinate.
Nasal steroids - Fluticasone and Triamcinolone.
Cough medicines - Guaifenesin.
Antihistamines - Fexofenadine and Loratadine.
Benefits of using OTC medicines
OTC medicines allow greater access to treatment of people at large at lower cost for minor or self-limiting illnesses.
Moreover, General Practitioners (GPs) do not have to write prescriptions for minor ailments, which gives them more time to deal with serious health problems.
This is extremely useful for countries like India, where the doctor-to-patient ratio is less (1:1800) than in other countries.
To ensure the optimum use of OTC medicines, pharmacists can provide a valuable interface by using their professional knowledge to guide patients. 5
Improved Education of the consumer.
Rapid access to effective medicines.
Allowing an individual to oversee their health.
Role of Pharmacist in OTC Medication Dispensing
Pharmacists play a vital role in controlling the number of medications being dispensed as OTC drugs. They are as follows,
Carefully read and follow all directions on the medicine bottle and box.
Dispense the minimum effective dose.
Call your doctor if you are having a problem with your medicines.
Do not give a medicine if the patient has had an allergic reaction.
Inform the patient to take the medicine exactly as directed.
Pharmacists must be careful when dispensing more than one drug.
Keep your doctor informed.
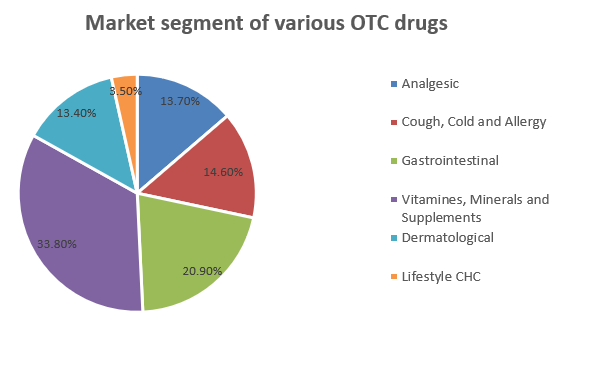
OTC drugs in India
The trend of 'Over-the-counter (OTC) Medicines' use has grown steadily in the last few years. This trend is responsible for various reasons, such as easy availability, affordability, and increased patient awareness. OTC or nonprescription medicines are terms used interchangeably to refer to medicines that can be bought without a prescription. Many countries recognize OTC medicines as a separate category of drugs and have established regulations for their use. In India, there are no guidelines for licensing OTC medicines. No separate category is allotted for OTC medicines in India, and the drugs that do not come under the prescription medicines schedule are generally sold as over-the-counter medicines. 6
Indian OTC market ranks 11th position in the global OTC market size, and it is expected to grow further due to more reasons: the expansion of more pharmaceutical companies and chemist shops in rural and semi-urban areas, people’s higher tendency to self-medicate, the need to save on health care spending. Increasing the trends in enhancing self-care activities and managing minor ailments through non-prescription drugs. 7
In India, medicines are listed under different schedules in the Drugs and Cosmetics Act and Rule. Drugs listed in Schedules H, H1, and X should carry a label stating that they are to be sold by retail only on the prescription of a registered medical practitioner. The drugs listed in Schedule G (mostly antihistamines) must carry a mandatory text on the label stating, “Caution: it is dangerous to take this preparation except under medical supervision.”
Surprisingly, specific important drug categories such as diuretics and aminosalicylates (Sulfasalazine, Mesalamine) are not included under any drug schedule. This confuses pharmacists about whether to sell these drugs as OTC or prescription medicines. 8 It must be stressed that the phrase “OTC” has no legal recognition in India. Here, the term OTC is used for how drugs are used (self-medication without prescription or allowed to be sold by pharmacists without a Registered Medical Practitioner) rather than being a recognized official category of medicines, unlike other countries.
The medicines that do not fall under Schedule H, H1, and X can be given without prescription through pharmacists and drugstores in India. Moreover, it is a common observation that prescription drugs are also sold without a prescription, like over-the-counter medicines. Ayurvedic drugs and traditional treatments are manufactured under a manufacturing license issued by the State Licensing Authorities.
These drugs are freely sold over the counter by non-pharmacists. Thus, there are no specific unifying regulations related to the use and sale of OTC products, and inappropriate use impacts both accessibility to better health care and patients' safety.
A study by Phalke et al., reported the prevalence of self-medication in the rural population of the state of Maharashtra in India to be 81.5%. However, in Tamil Nadu, only 23% of the rural population resorted to self-medication or household remedies, and almost 77% consulted a doctor. The prevalence of self-medication in Urban Delhi was found to be 92.8%, whereas a study conducted at Berhampur in Odisha found the prevalence of OTC medication use to be 18.72%. Overall, 52% of Indians were estimated to self-medicate in India according to a web portal-based survey of 20000 people across 10 cities. The reasons were lack of time, the need to avoid doctors' fees, and dependence on the Internet. One Indian study reported that more than 90% of the qualified pharmacists interviewed were aware of the concept of OTC drugs, 96.5% asked the patients their complaints when they were approached to purchase OTC drugs, but only 51% counselled the patients regarding instructions for use. 5
Table 1
Popular OTC Brands across variouscategories 11
India OTC Market Recent Developments
The top OTC products available in India include Dabur Honitus lozenges, cough syrups, Vicks VapoRub, Zandu balm, Iodex, Moov, cough drops, and lozenges. One of India's most significant economic sectors is the pharmaceutical industry, which exports $15 billion worth of goods yearly and boasts some of the world's best manufacturers.
OTC and prescription medicines are India's second-largest export to the US.
Bayer announced its intention to join India's OTC Drug market in June 2022. According to the company, Bayer's consumer health business in India, which recently introduced the new Saridon Advance for severe headaches, is scheduled to introduce another product in the nutrition category in July.
The Union Government suggested in May 2022 that the law should be altered to introduce OTC drugs into India according to the Drugs and Cosmetics Rules, enabling their retail sale without a medical prescription.
As a result of the development of technology and marketing, higher levels of workplace stress, and rising health awareness, urban India has picked up the idea of OTC drugs.
A few FMCG companies, including Hindustan Unliver and P&G, are expanding their markets by joining the OTC space and reaching previously inaccessible regions. Pharmacies' inability to get to the country's remote areas is one of the primary challenges they face.
India's Pharmaceutical sector makes up around 2.5% of the global pharmaceutical market in terms of value and 10% in terms of volume.
Continuous improvement innovation and rising investments in substantial research and development (R&D) efforts further drive the industry. Other factors affecting the marketplace include increasing disposable income levels among the public, growing stress levels among working professionals, and rising health consciousness among many citizens.
In April 2020, Cipla Health will transport over-the-counter wellness products to customers' homes in India in collaboration with Zomato, Swiggy, and Dunzo.
GSK Consumer Healthcare announced in December 2020 the launch of a novel nasal decongestant portfolio in India under the Otrivin brand. Additionally, Otrivin Breathe Clean Daily Nasal Wash was launched.
India OTC market top players
Emami limited
Dabur India Limited
Procter & Gamble
Abbott laboratories
GlaxoSmithKline
Cipla
Sun Pharmaceuticals Limited
Johnson & Johnson 10
Safety of OTC drugs: need for separate Pharmacovigilance system
OTC drugs are relatively safe. However, inappropriate use can lead to complications and adverse events that affect public health. The consumption of these medicines can be unsafe, as incorrect self-diagnosis or the ingestion of inappropriate doses can lead to side effects and the occurrence of adverse reactions and drug-drug interactions. Measures should be implemented to optimize the safe use of OTC drugs to avoid the occurrence of secondary events associated with the lack of knowledge related to their usage. Recently, an increase has been observed in the use of pharmaceutical drugs by the general population. However, this increase has not been associated with health improvement.
According to the World Health Organization (WHO), more than 50% of the drugs consumed worldwide are prescribed or dispensed inappropriately, and almost 50% of patients misuse these drugs, resulting in increased morbidity and mortality. This self-management of health encompasses the concept of self-medication, defined as the selection and use of drugs by individuals and caregivers to treat self-recognized or self-diagnosed conditions or symptoms. This self-medication can be an unsafe practice due to inaccurate self-diagnosis or an inappropriate dosage intake that can result in side effects, adverse reactions, and pharmacological interactions. Improper self-medication increases the costs associated with health care in the number of patients taking OTC. Increased access to medicines without improved health literacy increases the risk of misuse. 12
Inappropriate self-medication with OTC drugs can have serious repercussions (including death), especially in pregnant and nursing mothers, paediatric and geriatric populations, and patients with co-morbidities. 13 National health policies concerning the use of OTC drugs should be targeted at these populations and drug groups to optimize their use and avoid the occurrence of side effects associated with the lack of knowledge of the use of these drugs. In addition, these policies should improve e-health literacy, considering the increase in the number of people who use the internet or social networks as health resources to search for information. 12
Incorrect use of over-the-counter drugs might result in health and other drug-related issues. A drug-related problem (DRP) is an incident or scenario involving drug therapy that interferes with anticipated health results, either directly or indirectly. Pharmacists are highly trained professionals obligated to provide effective patient counselling to guarantee the safe use of pharmaceuticals. They play a vital and proactive role in preventing and resolving DRPs, thus preventing adverse events, avoiding extra costs resulting from inappropriate use of medications, and adding value to patient safety.
Pharmacists are the most accessible healthcare professionals on the front lines for the general population and, seemingly, the most equipped professionals with formal knowledge and training on self-treatment and over-the-counter pharmacotherapy. As a result, pharmacists in community settings have momentous responsibility and opportunity to respond to patients’ minor complaints and safeguard them from the risks associated with self-medication. Pharmaceutical counselling provided by community pharmacies is particularly crucial when medications are purchased over-the-counter (OTC) without advice given by a physician.
Easily accessible OTC medications are considered harmless by consumers and thus often underestimate the potential risks. They have an incomplete awareness of several risk areas of OTC medications, such as those relating to drug interactions and misuse/abuse 13
Consumers think that OTC medicines are safe since pharmacists dispense them without a prescription from a registered medical practitioner. However, OTC has adverse effects and could be misused, abused, or interact with prescription drugs. The patient must assess the safe use of these products without the practitioner’s intervention. Therefore, people must acquire knowledge about the safety of OTC medicines. Failure to comply with directions may lead to profound side effects (i.e., the correct dose, correct schedule, and for the intended disease). For example, an overdose of Acetaminophen has been associated with liver damage. Analgesics increase gastrointestinal tract bleeding even when taken at appropriate doses. Cough products such as Dextromethorphan and Diphenhydramine may be abused due to their euphoric and alcohol-like effects. 14
Over-the-counter Drugs Market in India
The market for OTC products in India can be categorized as (1) frank OTC products that are advertised on public media and construed as actual OTC products, (2) prescription brands that are not advertised but are promoted to physicians and also purchased by consumers without prescription called OTC brands.
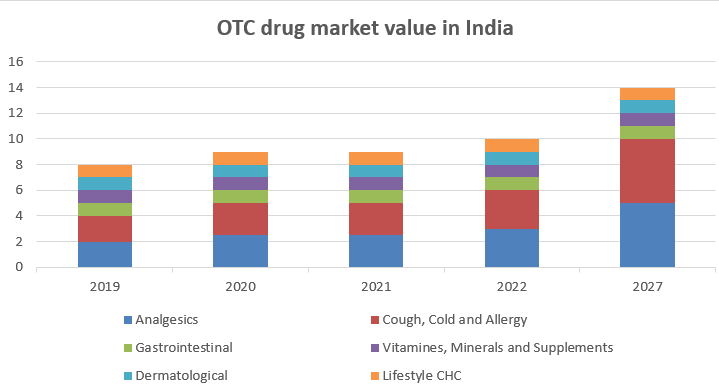
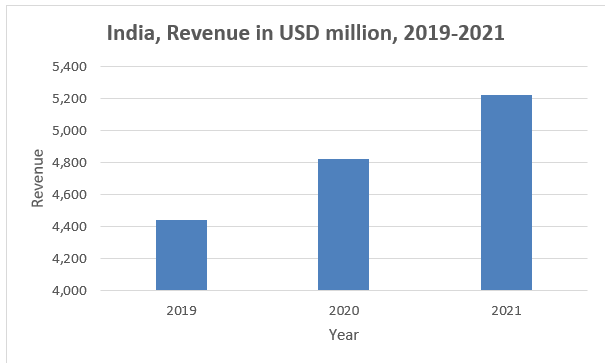
The value of the Indian OTC drugs market is estimated to be US$ 2.7 billion (Rs 188.6 billion) at a compounded annual growth rate of 9% to reach around $6.5 billion (Rs 441.1 billion).10
The significant players for OTC products in India are Cipla, Abbott India Limited, Amrutanjan Health Care Limited, Boehringer Ingelheim Limited, Mankind, Dabur India Limited, Pfizer,
Emami, GlaxoSmithKline, Sanofi, Himalaya Herbal Health Care, Novartis, Marico, Merck, Piramal Enterprises, Procter & Gamble, and Lupin.15
Table 2
Approved drugs in India16
Labeling of OTC drugs
Labeling criteria: Labeling is crucial for over-the-counter medicines. It provides consumers with important drug information, and the content should be easy to understand. According to Rule 95 of the D&C Act, labels must adhere to the requirements. The essential characteristics of over-the-counter medications will also be modified, and they will be classified as OTC-1 and OTC-2 based on safety, therapeutic index, patient accessibility requirements, availability, non-addictive nature, supply chain mechanism, and socio-demographic conditions of the nation. These changes will be made along with the definition and rules for advertising.15
Section 96 of DCR stipulates the labeling instructions. There are no specific labeling conditions or requirements for OTC drugs in India. In contrast, it is mandatory for all medicines except Ayurvedic, Siddha, and Unani drugs to put the following information on their labels:
Generic name and brand name
Contents of ingredients and total contents
Details of the manufacturer, including name, address, and manufacturing license number
The batch details, dates of manufacturing, and expiry dates
The maximum retail prices
Rule 161 states the labeling provisions of all Ayurvedic, Siddha, and Unani drugs 10
Indian Pharmaceutical Association Recommendations
The word OTC (over-the-counter) Medicines must be defined under the Act, giving it legal status.
In addition, we need to have two sub-categories of OTC medications:
Table 0
Non-prescription or otc medicines
Labelling
The medicine label should specify that it is an OTC Category Medicine, a green.
Vertical line of 1 mm thickness, and have the written box as below (and a symbol):
In addition to the above messages, the package should include one of the following:
The medicine package should compulsorily contain a PPI (Patient Product Information/Patient Package Insert/s) or a PIL (Patient Information Leaflet) (in a readable font) ‐in English and at least two local languages prevailing in the locality/state in which the medicine is sold. The drug control authority must approve this PPI or PIL, and the manufacturer must insert it.
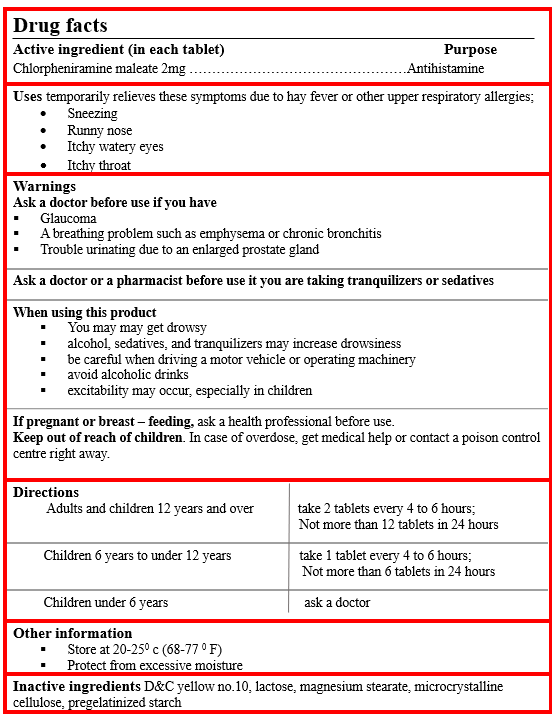
The medicine carton/label should bear the “Drug Facts.” The below-stated example is from the USA.
The information provided in these should cover the following:
Any warnings/contra‐indications for use.
Indications, dosage, frequency of dose, maximum daily dose.
Potential adverse effects (those which can be ignored, and those, if seen by the patient, should stop taking the medicine).
DO NOT use for more than three days/week without consulting your doctor or pharmacist.”
Please read the enclosed PPI (Patient Package Insert) before using the medicine.
Otc ‐ General Sales Medicines
These medicines can be sold at shops/general stores besides pharmacies. The medicine
label should specify that it is an OTC ‐ General Sales medicine has a specific
symbol designating it, a green vertical line of 2 mm thickness, and the written box
as below.
These medicines can be advertised to the public without making tall claims or misleading the public. All advertisements should also contain warning messages in bold and be easy to locate and read in English and at least two local languages.
Examples: Dettol, Savlon, Band-aids 17
Conclusion
The consumption of OTC drugs has increased worldwide. Consumers are buying OTC drugs without considering their side effects. The public widely practices self-medication.
Significant problems and malpractices are associated with sharing OTC medications, using expired medicines, doubling the dose of drugs when they are ineffective, storing OTC medications, and not reading labels and expiry dates.
As the selection and use of over-the-counter medications are continuously increasing, pharmaceutical counseling should be readily available and actively provided for consumers to achieve safer self-medication. Improved pharmacists’ practices about OTC can improve the rational use of non-prescription (OTC) drugs. Pharmacists must remain updated in their knowledge to make such practice safe and valuable.
There are no guidelines for OTC medications in India regarding labeling specific OTC categories. There is a critical need to improve and organize the labeling of OTC medicines so they are simple, straightforward, and easy to read. Drug regulatory authorities in India should enforce stringent policies in respective states so that practicing community pharmacists only dispense safe medications such as OTC drugs.
There is a need to create awareness about OTC drugs among various categories of communities, especially in rural areas. Orientation programs are needed to conduct, which can help reduce misuse and abuse and elicit proper use of medications.
A legally recognized class of OTC pharmaceuticals, patient awareness programs, and the support of pharmacists and pharmaceutical companies are required to maximize the rational usage of OTC medications in India. The Ministry of Health in India should control regulations governing the manufacture of patient information booklets in a local language for easy comprehension. National health policies concerning the use of OTC drugs should be targeted at these populations and drug groups to optimize their use and avoid the occurrence of side effects associated with the lack of knowledge of the use of these drugs. In addition, these policies should improve e-health literacy, considering the increase in the number of people who use the internet or social networks as health resources to search for information.
It is recommended that more information about the risks of self-medication, drug adverse reactions, antibiotic stewardship, more supervision of the prohibition of over-the-counter drugs and selling practices, and adequate facilities for people’s access to medical services be provided at the policy level.
Hence, a separate pharmacovigilance system should be implemented to optimize the safe use of OTC drugs and avoid secondary events associated with a lack of knowledge about their usage.