Introduction
Biodegradation is a natural process where organic chemicals in the environment are broken down into simpler compounds, mineralized, and redistributed through elemental cycles like the carbon, nitrogen, and sulphur cycles. Biodegradable polymers are extensively used in biomedical applications due to their biocompatibility and biodegradability. These polymers are designed for temporary applications such as sutures, tissue-supporting scaffolds, and drug delivery devices 1 They maintain their properties for a limited period before gradually degrading into soluble molecules that can be excreted from the body.2
Biodegradable polymers are favoured for drug delivery applications because they eliminate the need for surgical removal of the depleted device. Despite the large number of biodegradable polymers, only a few are suitable for drug delivery applications.3 These suitable candidates must not only be biodegradable but also meet high biocompatibility standards. Additionally, they should offer processability, sterilizability, and storage stability to be useful in biomedical applications. The primary advantage of degradable polymers is that they break down into biologically acceptable molecules that are metabolized and removed from the body via normal metabolic pathways. However, biodegradable materials produce degradation by-products that must be tolerated with minimal or no adverse reactions within the biological environment. These degradation products, both desirable and potentially undesirable, must be thoroughly tested, as various factors can affect the biodegradation of the original materials. The most important factors are listed below, providing an indication of the range of structural, chemical, and processing properties that can influence biodegradable drug delivery systems.
Factor affecting biodegradation of polymers
Chemical structure
Chemical composition
Distribution of repeat units in multimers
Presents of ionic groups
Presence of unexpected units or chain defects
Configuration structure
Molecular weight
Molecular-weight distribution
Morphology (amorphous/semicrystalline, microstructures, residual stresses).
The presence of low-molecular-weight compounds
Processing conditions
Annealing
Sterilization process
Storage history
Shape
Site of implantation
Adsorbed and absorbed compounds (water, lipids, ions, etc
Physicochemical factors (ion exchange, ionic strength, pH
Physical factors (shape and size changes, variations of diffusion coefficients, mechanical stresses, stress- and solvent-induced cracking, etc.)
Mechanism of hydrolysis (enzymes versus water)
Biodegradable polymers used in drug delivery applications are derived from either natural or synthetic sources.
While the use of these polymeric materials has significantly advanced modern healthcare, concerns about their long-term biocompatibility have persisted.4 In the latter half of the twentieth century, material scientists began engineering novel polymeric materials or modifying existing ones to achieve biocompatibility and adequate mechanical properties for specific biomedical applications. Polymers initially developed for biodegradable sutures have proven effective and successful for long-term drug delivery applications.
Recently, there has been rapid growth in drug discovery, driven by novel technologies such as combinatorial chemistry and high-throughput screening. These approaches have led to the development of drugs that are generally more potent but have poorer solubility compared to those developed through traditional medicinal chemistry. Consequently, there is an increased focus on developing novel techniques to deliver these complex drugs more effectively and efficiently.
Table 1
Shows some of the synthetic polymeric materials employed and their biomedical applications.
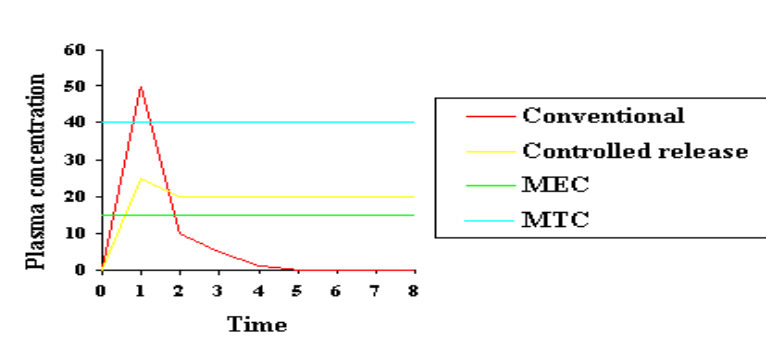
As illustrated in Figure 1, conventional oral and intravenous drug administration routes often fail to provide ideal pharmacokinetic profiles, particularly for drugs with high toxicity or narrow therapeutic windows. 6 For these drugs, the optimal pharmacokinetic profile would involve reaching therapeutic levels without exceeding the maximum tolerable dose and maintaining these levels for extended periods until the desired therapeutic effect is achieved. One way to achieve such a profile is by encapsulating the drug in a polymeric matrix. Over the past 30 years, the technology of polymeric drug delivery has been extensively studied, with numerous excellent reviews available. Biodegradable polymers are particularly desirable in these scenarios because they degrade into biologically inert and compatible molecules within the body. By incorporating drugs into biodegradable polymers, dosage forms that release the drug over a prolonged period can be created in various shapes and sizes. No surgical procedures are required after the dosage regimen is complete, as the remaining polymer will degrade and be cleared by the body. Consequently, biodegradable polymers offer a novel approach for developing sustained-release drug delivery systems that are simple and convenient for patients.
The three key advantages of polymeric drug delivery products are
Localized delivery of the drug: The product can be implanted directly at the site where drug action is needed, reducing systemic exposure to the drug. This is especially important for toxic drugs associated with various systemic side effects, such as chemotherapeutic drugs.
Sustained delivery of drugs: The encapsulated drug is released over extended periods, eliminating the need for multiple injections. This feature can improve patient compliance, particularly for drugs used in chronic conditions that require frequent injections, such as certain protein deficiencies.
Stabilization of the drug: The polymer can protect the drug from the physiological environment, improving its stability in vivo. This feature makes this technology attractive for delivering labile drugs, such as proteins.
As illustrated in Figure 2, drug release over time can occur either through diffusion out of the polymer matrix or by degradation of the polymer backbone. This continuous release could potentially achieve a pharmacokinetic profile close to the ideal scenario depicted in Figure 1. 6 Recent advances in biotechnology and pharmaceutical science have opened new frontiers in biomedical fields, demanding materials with bioactivity, biocompatibility, and often transient existence. Such transient materials are highly preferred for in vivo applications like implantable drug delivery systems and conduits for guiding or remodelling damaged tissues.7 The continuous release of drugs from the polymer matrix can occur through diffusion, polymer erosion (due to degradation), or a combination of both mechanisms. Several reviews have discussed the mechanisms and mathematical aspects of drug release from polymer matrices. For a given drug, the release kinetics from the polymer matrix are primarily governed by three factors: the polymer type, polymer morphology, and the excipients present in the system. The following sections will focus on these factors and their roles in the drug release characteristics of a polymeric system.
Synthetic Biodegradable Polymers
Various synthetic biodegradable polymers are currently being investigated as drug delivery systems or scaffolds for tissue engineering.7 The advent of biodegradable polymers has significantly influenced the development and rapid growth of various technologies in modern medicine. These polymers are mainly used where transient existence is required, finding applications as sutures, scaffolds for tissue regeneration, tissue adhesives, hemostats, transient barriers for tissue adhesion, and drug delivery systems. Each application demands materials with unique physical, chemical, biological, and biomechanical properties to provide efficient therapy. Consequently, a wide range of degradable polymers, both natural and synthetic, have been investigated for these applications. However, the composition of natural polymers can vary from source to source.
Advantages of Biodegradable Polymers as Drug Carriers
Polymers are large molecules composed of many smaller units called monomers bonded together. In addition to eliminating the need for removal, the five key advantages of polymeric drug delivery products are:
Localized delivery of the drug: The product can be implanted directly at the site where drug action is needed, reducing systemic exposure. This is especially important for toxic drugs associated with various systemic side effects, such as chemotherapeutic drugs.
Sustained delivery of drugs: The encapsulated drug is released over extended periods, eliminating the need for multiple injections. This feature can improve patient compliance, particularly for drugs used in chronic conditions requiring frequent injections, such as certain protein deficiencies.
Stabilization of the drug: The polymer can protect the drug from the physiological environment, improving its stability in vivo. This feature makes this technology attractive for delivering labile drugs, such as proteins.
Release rate less dependent on drug properties: In diffusion-controlled systems, the release rate typically declines over time. However, a biodegradable system may yield a constant release even with a simple monolithic device if matrix degradation can compensate for this decline, possibly with an increase in drug permeability. 8
Steady release rate over time: Biodegradable systems can maintain a steady release rate, which is beneficial for achieving consistent therapeutic effects.
The degradation of lactide-based polymers, and hydrolytically degradable polymers in general, depends on the following properties:
Chemical composition: The rate of degradation depends on the type of degradable bonds present in the polymer. Generally, the degradation rate follows the order: Anhydride > Esters > Amides.
Crystallinity: Polymers with higher crystallinity degrade more slowly
Hydrophilicity: Polymers with many hydrophobic groups degrade more slowly than hydrophilic polymers 9
Drug Release Mechanisms
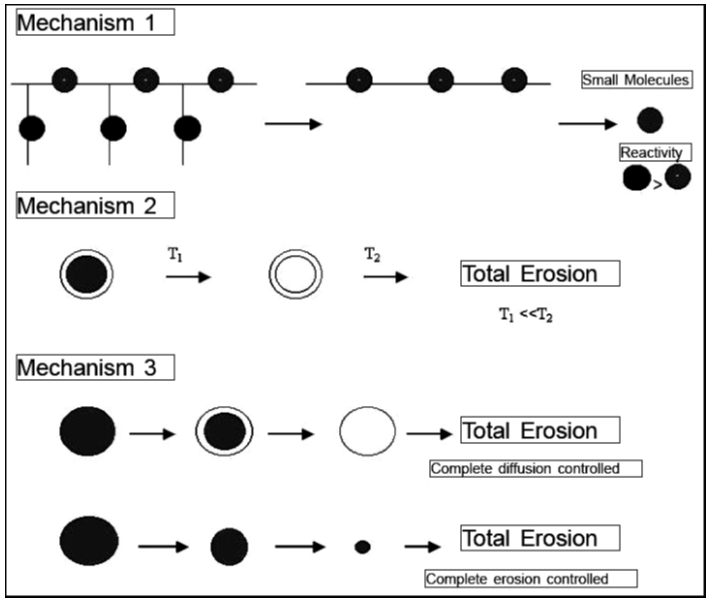
The release of drugs from erodible polymers can occur through several mechanisms, as illustrated in Figure 3:
Mechanism 1: The drug is attached to the polymeric backbone via a labile bond, which has a higher reactivity towards hydrolysis than the polymer itself. This allows the drug to be released as the bond breaks down.
Mechanism 2: The drug is encapsulated in a core surrounded by a biodegradable, rate-controlling membrane. This reservoir-type device ensures that the drug is released gradually and eliminates the need for surgical removal once the drug is depleted.
Mechanism 3: The drug is homogeneously dispersed within the biodegradable polymer. The release occurs through erosion, diffusion, or a combination of both processes.9
These mechanisms enable controlled and sustained drug release, enhancing the effectiveness and convenience of drug delivery systems.
Figure 3
Schematic representation of drug release mechanisms in mechanism 1, drug is released by hydrolysis of polymeric bond. In mechanism 2, drug release is controlled by biodegradable membrane. In mechanism 3, drug is released by erosion, diffusion, or a combination of both.10
Polymer Morphology
The morphology of the polymer matrix plays a crucial role in determining the release characteristics of the encapsulated drug. The polymer matrix can be formulated in various forms, such as micro/nano-spheres, gels, films, or extruded shapes like cylinders and rods. The shape of the extruded polymer can significantly impact drug release kinetics. For instance, zero-order drug release can be achieved using a hemispherical polymer form.
Polymer microspheres are the most popular form due to their manufacturing advantages and ease of administration (injectability by suspending in a vehicle). As shown in Figure 2, polymer microspheres can be produced using various techniques such as spray drying and solvent evaporation. The chosen technique affects factors like porosity, size distribution, and surface morphology of the microspheres, which in turn influence the performance of the drug delivery product.
Polymeric drug delivery products can also be formulated with excipients added to the polymer matrix. The main objectives of incorporating excipients are to modulate drug release, stabilize the drug, or adjust the polymer degradation kinetics. Recent studies by Schwendeman and colleagues have demonstrated that incorporating basic salts as excipients in polymeric microspheres can improve the stability of the incorporated protein. However, these basic salts also slow down the degradation of the polymer. Similarly, hydrophilic excipients can accelerate drug release, although they may also increase the initial burst effect. 10
Controlled-Release Methodologies
Controlled-release methodologies can be classified based on the mechanism that controls the release of the active agent from the delivery device: diffusion, osmosis, or polymer erosion. There are three basic types of polymer erosion mechanisms:
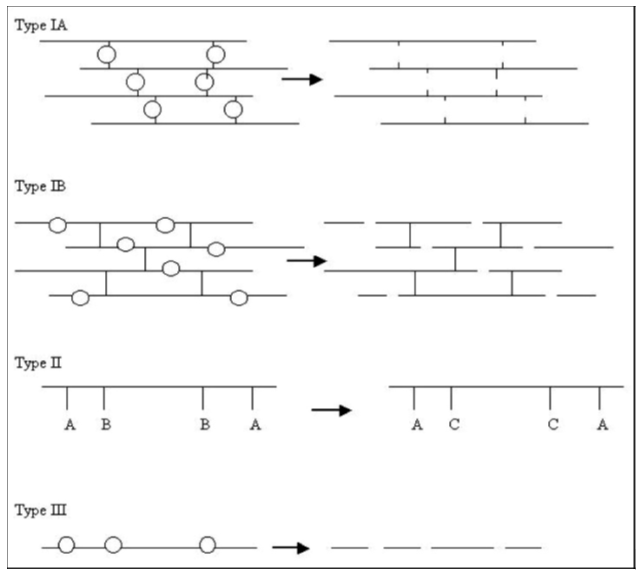
Type I Erosion: This involves water-soluble polymers that have been insolubilized by covalent cross-links. These polymers solubilize as the cross-links (Type IA), or the backbone (Type IB) undergo hydrolytic cleavage.
Type II Erosion: Initially water-insoluble polymers become soluble through hydrolysis, ionization, or protonation of a pendant group.
Type III Erosion: Hydrophobic polymers are converted into small water-soluble molecules by backbone cleavage
The choice of erosion mechanism depends on the specific application. Various systems utilize these mechanisms:
Reservoir Systems: Drugs diffuse from a reservoir through a bioerodible membrane
Microcapsules: These encapsulate the drug within a biodegradable shell
Monolithic Devices: The drug is uniformly dispersed within the polymer matrix
Chemically Bound Systems: Drugs are chemically bound to a bioerodible polymer
There are two primary mechanisms for drug release from bioerodible polymers:
Rate-Controlling Membrane: The drug core is surrounded by a bioerodible membrane that controls the release rate.
Monolithic Device: The drug is dispersed within the polymer, forming a bioerodible monolithic device
For the sustained release of fertility-regulating agents, Type III erosion is often used. Polymer erosion typically leads drug release, and there is evidence that drug release from the implant is controlled by the solubilization rate of the highly water-insoluble steroid.
In some contexts, ‘biodegradation’ refers specifically to chemical processes that alter the molecular weight or solubility of a polymer, while ‘bioerosion’ is used to describe physical processes that result in the weight loss of a polymer device. A polymer that can degrade and have its by-products assimilated or excreted by a living system is termed bioresorbable.
Erosion typically occurs in devices made from soluble polymers. In these cases, the device erodes as water is absorbed, causing the polymer chains to hydrate, swell, disentangle, and eventually dissolve. Alternatively, degradation can result from chemical changes to the polymer, such as cleavage of covalent bonds, ionization, and protonation along the polymer backbone or on pendant side chains. General factors affecting the biodegradation of polymers are listed in [Table 2]. The erosion mechanism of polymers can be described both physically and chemically.
Chemical Erosion: According to Heller, there are three general chemical mechanisms that cause bioerosion.
Table 2
Classificationof biodegradable polymers following the heller terms
Mechanism I: Degradation of Water-Soluble Macromolecules
In this mechanism, water-soluble macromolecules are crosslinked to form a three-dimensional network. As long as the crosslinks remain intact, the network is insoluble. Degradation can occur in two ways:
Type IA: Degradation at the crosslinks, resulting in soluble backbone polymeric chains. This typically produces high molecular weight, water-soluble fragments.
Type IB: Degradation at the main chain, leading to the formation of water-soluble fragments. This generally results in low molecular weight, water-soluble oligomers and monomers.hese processes are illustrated in Figure 4
Figure 4
Schematic representation of degradation mechanisms A - hydrophobic substituent, B to C - represent hydrolysis, ionization or protonation. Type I describes the degradation of water-soluble macromolecules. Type II describes the dissolution of water insoluble macromolecules. Type III describes the degradation of insoluble polymers with labile bonds10
Mechanism II: Dissolution of Water-Insoluble Macromolecules
In this mechanism, water-insoluble macromolecules with side groups are converted into water-soluble polymers through ionization, protonation, or hydrolysis of these groups. The polymer itself does not degrade, and its molecular weight remains essentially unchanged. Examples of materials exhibiting type II erosion include cellulose acetate derivatives and partially esterified copolymers of maleic anhydride. These polymers become soluble through the ionization of carboxylic groups. 8
Mechanism III: Degradation of Insoluble Polymers with Labile Bonds
This mechanism involves the degradation of insoluble polymers with labile bonds. Hydrolysis of these labile bonds leads to the scission of the polymer backbone, resulting in low molecular weight, water-soluble molecules. Polymers such as poly (lactic acid), poly (glycolic acid) and their copolymers, poly(ortho esters), polyamides, poly(alkyl-2-cyanoacrylates), and polyanhydrides undergo type III erosion. These three mechanisms are not mutually exclusive and can occur in combination. 8
Physical erosion
Physical erosion mechanisms can be classified as either heterogeneous or homogeneous:
Heterogeneous Erosion (Surface Erosion): In this type, the polymer erodes only at the surface while maintaining its physical integrity as it degrades. This allows for predictable drug kinetics, and zero-order release kinetics can be achieved by applying the appropriate geometry. Highly crystalline polymers tend to undergo heterogeneous erosion because crystalline regions exclude water. However, few polymers exhibit this type of erosion.
Homogeneous Erosion: In this type, hydrolysis occurs at an even rate throughout the polymeric matrix. These polymers are generally more hydrophilic than those exhibiting surface erosion, allowing water to penetrate the matrix and increase the rate of diffusion. Homogeneous erosion results in the loss of integrity of the polymer matrix. Synthetic condensation polymers are generally biodegradable to varying extents, depending on factors such as chain coupling (ester > ether > amide > urethane), morphology (amorphous > crystalline), molecular weight (lower > higher), and hydrophilicity (hydrophilic degrades faster than hydrophobic). However, it is important to note that water solubility does not necessarily imply biodegradability. 8
Recent Developments in the Use of Polymers for Drug Delivery Systems
Oral drug delivery has long been the most widely utilized route for systemic drug delivery, largely due to its ease of administration. 6
Both natural and synthetic polymers have been extensively studied for their potential applications in drug delivery. Synthetic polymers offer advantageous properties and a wide range of options. Two promising synthetic polymers developed for biomedical applications are polyvinylpyrrolidone and polyethylene glycol acrylate-based hydrogels. These polymers are biodegradable and can form copolymers with natural macromolecules.
Natural polymers, on the other hand, are highly biocompatible and less immunogenic. Notable natural polymers include collagen and Gelatin, as well as chitosan, alginate, starch, pectin, casein, and cellulose derivatives. Composites of these natural polymers with synthetic polymers offer additional advantages as drug delivery carriers by complementing each other’s properties.
Researchers have developed collagen poly-HEMA hydrogels in the laboratory as implants for delivering anticancer drugs such as 5-fluorouracil, mitomycin, and bleomycin for solid fibrosarcoma in rat models. These hydrogels have also been modified for the delivery of model proteins like bovine serum albumin and vaccines such as tetanus and diphtheria toxoids in mice. Additionally, hybrid copolymers of collagen with biodegradable synthetic polymers like polyethylene glycol 6000 and polyvinylpyrrolidone have been developed for the controlled release of contraceptive steroids.11
Polylactides are more hydrophobic compared to PLGA and take longer to degrade. Among polylactides, DL-PLA (a polymer of D and L-lactide) degrades faster than L-PLA (a homopolymer of L-lactide), likely due to its lower crystallinity. Similarly, the more hydrophobic end-capped PLGA polymers degrade faster than carboxyl-ended PLGA.
Despite the numerous advantages of PLA and PLGA-based polymers, their commercialization faces certain challenges. One major concern is the existence of over 500 patents for various applications of these polymers, which raises the risk of patent infringement when developing new products. Additionally, PLA and PLGA polymers have inherent limitations in terms of flexibility and stability. To address these issues, researchers are exploring several new polymers for drug delivery applications. Some of the new polymers in clinical or preclinical development include polyorthoesters, polyphosphazenes, polyanhydrides, and polyphosphoesters. 7 Researchers are also investigating copolymers of PLGA to overcome its limitations.
Conclusion
Polymer-based pharmaceuticals are increasingly recognized as crucial in treating serious diseases such as cancer and hepatitis. Traditionally, excipients in formulations were considered inert substances used to add volume and aid in manufacturing. However, they are now being included in dosage forms to perform specialized functions that enhance drug delivery, especially for new drugs with unfavorable physicochemical and pharmacokinetic properties. Synthetic polymers can be tailored or modified to meet specific formulation requirements by altering their characteristics. In contrast, natural pharmaceutical excipients are biocompatible, non-toxic, environmentally friendly, and cost-effective. Numerous polymers have been successfully utilized, and many others are under investigation as excipients in the design of effective drug delivery systems.