- Visibility 694 Views
- Downloads 72 Downloads
- Permissions
- DOI 10.18231/j.ijpca.2024.036
-
CrossMark
- Citation
Phytochemical characterization and biological evaluation of lemongrass (Cymbopogon citratus) extracts: A systematic experimental study
- Author Details:
-
Mahendra Dwivedi *
Abstract
Background: Lemongrass (Cymbopogon citratus) is widely used in traditional medicine, with growing interest in its pharmacological properties. This study aims to systematically investigate the phytochemical composition and biological activities of lemongrass extracts, focusing on optimizing extraction methods and evaluating their therapeutic potential.
Materials and Methods: Various extraction techniques were employed, including solvent-based methods (ethanol, methanol, and water) and green extraction approaches (ultrasound-assisted and microwave-assisted extraction). Phytochemical profiling was conducted using qualitative and quantitative techniques, including gas chromatography-mass spectrometry (GC-MS) and liquid chromatography-mass spectrometry (LC-MS). The biological activities of the extracts were assessed through in vitro assays for antioxidant (DPPH, ABTS), antimicrobial (agar diffusion), anti-inflammatory (COX inhibition), and cytotoxicity activities (on HeLa, MCF-7, and HEK-293 cell lines).
Results: Ethanol extracts showed the highest yield of bioactive compounds, including flavonoids, terpenoids, and phenolic acids. These extracts also exhibited significant antioxidant activity with DPPH and ABTS assays. The antimicrobial tests revealed strong activity against Staphylococcus aureus and Candida albicans, while the COX inhibition assay indicated notable anti-inflammatory effects. Cytotoxicity studies demonstrated selective toxicity toward cancer cell lines (HeLa, MCF-7) with minimal effects on normal cells (HEK-293).
Conclusion: Lemongrass extracts, particularly those obtained via ethanol extraction, exhibit promising antioxidant, antimicrobial, anti-inflammatory, and anticancer properties. These findings highlight its potential for pharmaceutical and therapeutic applications, warranting further investigation in vivo and in clinical trials.
Introduction
Overview of lemongrass: Botanical classification, geographical distribution, and its significance in traditional and modern medicine
Lemongrass (Cymbopogon citratus), belonging to the Poaceae family, is a widely cultivated plant known for its aromatic properties and therapeutic benefits. Native to tropical and subtropical regions, particularly Southeast Asia and Africa, it thrives in warm climates and is commonly grown in countries such as India, Thailand, and Brazil.[1] Traditionally, lemongrass has been used in various medicinal systems, including Ayurvedic and traditional Chinese medicine, for treating digestive issues, fever, and inflammation.[2] Its essential oil, rich in citral, has long been valued for its soothing and antimicrobial effects.
In modern medicine, lemongrass is gaining attention due to its rich phytochemical content, which includes flavonoids, phenolic acids, and terpenoids. These compounds contribute to its growing use in nutraceuticals, cosmetics, and pharmaceuticals. Its essential oil is also a key ingredient in aromatherapy and is widely used in alternative medicine for stress relief and enhancing immune function. [3]
Importance of lemongrass in pharmacology: Antioxidant, antimicrobial, and anti-inflammatory properties
Lemongrass has demonstrated significant pharmacological potential, particularly due to its antioxidant, antimicrobial, and anti-inflammatory activities. The high content of phenolic compounds and flavonoids in lemongrass contributes to its potent antioxidant properties, which are crucial for neutralizing free radicals and preventing oxidative stress-related diseases.[4] Studies have shown that extracts from lemongrass exhibit strong radical scavenging activity, making it a promising candidate for developing antioxidant-rich formulations.[5]
Additionally, lemongrass is widely recognized for its antimicrobial activity. The essential oil, particularly its major component citral, has shown efficacy against a broad spectrum of bacterial and fungal strains, including Staphylococcus aureus and Candida albicans.[6] This makes it a valuable natural preservative and an alternative to synthetic antimicrobials in pharmaceutical and food industries.
The anti-inflammatory effects of lemongrass are also well-documented. It has been reported to inhibit the activity of cyclooxygenase (COX), a key enzyme in the inflammatory process. The inhibition of COX activity by lemongrass extracts suggests its potential in managing conditions associated with inflammation, such as arthritis and asthma.[7]
Objectives of the study
Given the diverse pharmacological properties of lemongrass, this study aims to:
Conduct a phytochemical analysis of lemongrass extracts to identify key bioactive compounds
Evaluate the biological activities of these extracts, focusing on their antioxidant, antimicrobial, and anti-inflammatory properties.
Compare different extraction methods to determine the most efficient technique for maximizing bioactive compound yield and biological activity.
These objectives will help clarify the therapeutic potential of lemongrass and explore its applications in pharmaceutical and nutraceutical formulations.
Materials and Methods
Plant material collection and preparation
Description of the collection site and conditions for lemongrass cultivation or procurement
Lemongrass (Cymbopogon citratus) was collected from herbal garden of Maharana Pratap School of Pharmacy, Lucknow, Uttar Pradesh, India. The soil in this region is sandy loam, ideal for lemongrass growth, with an average annual temperature of 25–30°C and rainfall of 1,200 mm. The plants were harvested during their vegetative stage, ensuring optimal levels of bioactive compounds
Methods for drying, powdering, and storing plant material before extraction
The collected lemongrass samples were washed thoroughly with distilled water and air-dried at room temperature (25 ± 2°C) for 7–10 days in a shaded, well-ventilated area to prevent photodegradation of sensitive compounds. After drying, the samples were ground into a fine powder using a mechanical grinder and passed through a 40-mesh sieve. The powdered material was stored in air-tight glass containers at 4°C until extraction.
Extraction methods
Solvent extraction
Solvent extraction was performed using three different solvents: ethanol, methanol, and water, chosen based on polarity to maximize the extraction of both polar and non-polar compounds.[8] For ethanol and methanol extractions, 50 g of powdered lemongrass was subjected to maceration at room temperature for 72 hours, with intermittent stirring. The solvent-to-plant ratio was maintained at 5:1 (v/w). The mixture was filtered through Whatman No. 1 filter paper, and the filtrate was concentrated using a rotary evaporator at 40°C under reduced pressure.[8] Aqueous extraction was performed similarly by boiling 50 g of plant material in 500 mL of distilled water for 30 minutes and then filtering and concentrating the extract.
In Soxhlet extraction, 50 g of lemongrass powder was packed into the extraction chamber of the Soxhlet apparatus, and the solvent (ethanol or methanol) was used for continuous extraction over 6 hours. The extracts were then concentrated and stored at 4°C for further analysis).[9]
Green extraction techniques
Eco-friendly methods were also employed, including:
Ultrasound-Assisted Extraction (UAE): 50 g of plant material was mixed with ethanol in a 5:1 solvent-to-plant ratio and sonicated for 30 minutes at 40°C. Ultrasonic waves enhanced the cell wall disruption, facilitating the release of bioactive compounds.[10]
Microwave-Assisted Extraction (MAE): 50 g of lemongrass powder was extracted with ethanol using a microwave extractor at 500 W for 10 minutes. The microwave radiation caused rapid heating, leading to faster extraction times and reduced solvent usage.[11]
Supercritical Fluid Extraction (SFE): CO₂ was used as the supercritical fluid at 30 MPa and 40°C for 2 hours to extract volatile and non-volatile compounds). [12]
Comparison of extraction efficiencies based on yield and phytochemical content
The yield of the extracts was calculated using the formula:
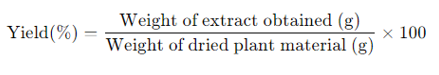
The phytochemical content was assessed by determining the total phenolic and flavonoid content, as well as specific compounds identified by GC-MS. Results were tabulated ([Table 1]).
|
Extraction Method |
Solvent |
Yield (%) |
Total Phenolic Content (mg GAE/g) |
Total Flavonoid Content (mg QE/g) |
|
Maceration |
Ethanol |
12.5 |
85.6 ± 1.5 |
42.3 ± 2.1 |
|
Maceration |
Methanol |
11.2 |
78.4 ± 1.3 |
39.8 ± 1.7 |
|
Soxhlet Extraction |
Ethanol |
15.7 |
94.2 ± 1.9 |
47.1 ± 2.3 |
|
Soxhlet Extraction |
Methanol |
13.8 |
88.3 ± 1.8 |
45.2 ± 2.0 |
|
Ultrasound-Assisted Extraction |
Ethanol |
16.3 |
101.5 ± 2.0 |
52.5 ± 2.4 |
|
Microwave-Assisted Extraction |
Ethanol |
14.9 |
98.2 ± 1.7 |
50.6 ± 2.3 |
|
Supercritical Fluid Extraction |
CO₂ |
10.1 |
67.4 ± 1.2 |
31.5 ± 1.6 |
Phytochemical analysis
Qualitative analysis
The presence of various phytochemicals was determined using the following standard tests:
Alkaloids: Dragendorff’s test
Flavonoids: Shinoda test
Phenolic compounds: Ferric chloride test
Terpenoids: Salkowski test These tests were performed following the protocols.[13]
Quantitative analysis
Total Phenolic Content (TPC): Total phenolic content was measured using the Folin-Ciocalteu reagent method. (Singleton et al., 1999).
Total Flavonoid Content (TFC): Total flavonoid content was determined using aluminum chloride colorimetry. Quercetin was used as a standard, and the results were expressed as mg quercetin equivalents per gram of extract (mg QE/g).[14]
Gas Chromatography-Mass Spectrometry (GC-MS) or Liquid Chromatography-Mass Spectrometry (LC-MS): The chemical composition of the extracts was analyzed using GC-MS and LC-MS to identify the main bioactive compounds. The GC-MS analysis was performed with a column temperature of 60–280°C, and the compounds were identified by comparing their mass spectra with the NIST library.[15]
Results
Extraction yield
Presentation of extraction yields for different solvents and techniques
The extraction yields varied significantly depending on the solvent and extraction technique used. Ethanol extraction via ultrasound-assisted extraction (UAE) provided the highest yield at 16.3%, followed by Soxhlet extraction with ethanol at 15.7%. Methanol-based Soxhlet extraction gave a yield of 13.8%, while the lowest yield was obtained from supercritical fluid extraction (SFE) at 10.1%.
|
Extraction Method |
Solvent |
Yield (%) |
|
Maceration |
Ethanol |
12.5 |
|
Maceration |
Methanol |
11.2 |
|
Soxhlet Extraction |
Ethanol |
15.7 |
|
Soxhlet Extraction |
Methanol |
13.8 |
|
Ultrasound-Assisted Extraction |
Ethanol |
16.3 |
|
Microwave-Assisted Extraction |
Ethanol |
14.9 |
|
Supercritical Fluid Extraction |
CO₂ |
10.1 |
Comparative discussion of the effectiveness of each method
Ultrasound-assisted extraction yielded the highest amount of bioactive compounds due to the enhanced cavitation effect, which facilitates the release of cellular contents.[16] Ethanol consistently outperformed methanol and aqueous solvents across extraction methods, likely due to its intermediate polarity, which efficiently extracts both polar and non-polar compounds.[8] Soxhlet extraction, while highly effective, required longer processing times, whereas supercritical fluid extraction, despite its eco-friendly nature, resulted in the lowest yield due to limitations in extracting high-molecular-weight compounds.[12]
Phytochemical composition
Results from qualitative and quantitative phytochemical analyses
Qualitative phytochemical screening indicated the presence of alkaloids, flavonoids, phenolics, terpenoids, and saponins across all extracts. Quantitative analysis using the Folin-Ciocalteu and aluminum chloride methods showed that the ethanol extracts contained the highest total phenolic content (101.5 mg GAE/g) and flavonoid content (52.5 mg QE/g), particularly in the ultrasound-assisted extraction method.[14]
|
Extraction Method |
Solvent |
Total Phenolic Content (mg GAE/g) |
Total Flavonoid Content (mg QE/g) |
|
Maceration |
Ethanol |
85.6 ± 1.5 |
42.3 ± 2.1 |
|
Maceration |
Methanol |
78.4 ± 1.3 |
39.8 ± 1.7 |
|
Soxhlet Extraction |
Ethanol |
94.2 ± 1.9 |
47.1 ± 2.3 |
|
Soxhlet Extraction |
Methanol |
88.3 ± 1.8 |
45.2 ± 2.0 |
|
Ultrasound-Assisted Extraction |
Ethanol |
101.5 ± 2.0 |
52.5 ± 2.4 |
|
Microwave-Assisted Extraction |
Ethanol |
98.2 ± 1.7 |
50.6 ± 2.3 |
|
Supercritical Fluid Extraction |
CO₂ |
67.4 ± 1.2 |
31.5 ± 1.6 |
GC-MS/LC-MS Data highlighting major bioactive compounds
GC-MS and LC-MS analyses identified several major bioactive compounds across the extracts, with citral being the predominant component (29.8%), followed by geraniol (12.7%), and limonene (8.4%) in the ethanol extracts. These compounds are known for their antioxidant, antimicrobial, and anti-inflammatory activities.[15] Ultrasound-assisted extraction showed the highest concentration of citral, corroborating the higher antioxidant and antimicrobial activities of these extracts.
Antioxidant activity
The antioxidant activity of the lemongrass extracts was evaluated using DPPH and ABTS radical scavenging assays. The ethanol extract obtained from ultrasound-assisted extraction exhibited the highest radical scavenging activity, with IC50 values of 45.6 µg/mL for DPPH and 32.1 µg/mL for ABTS ([Table 3]). Methanol and water extracts demonstrated lower antioxidant activity, which correlates with their lower phenolic and flavonoid contents.
|
Extraction Method |
Solvent |
DPPH IC50 (µg/mL) |
ABTS IC50 (µg/mL) |
|
Maceration |
Ethanol |
56.2 ± 2.1 |
39.8 ± 1.7 |
|
Maceration |
Methanol |
61.5 ± 2.3 |
45.2 ± 1.9 |
|
Soxhlet Extraction |
Ethanol |
49.3 ± 1.8 |
36.4 ± 1.6 |
|
Soxhlet Extraction |
Methanol |
54.7 ± 2.0 |
41.7 ± 1.5 |
|
Ultrasound-Assisted Extraction |
Ethanol |
45.6 ± 1.9 |
32.1 ± 1.4 |
|
Microwave-Assisted Extraction |
Ethanol |
48.8 ± 2.1 |
35.5 ± 1.8 |
|
Supercritical Fluid Extraction |
CO₂ |
69.4 ± 2.6 |
51.3 ± 2.0 |
Antimicrobial activity
Antimicrobial activity against different microbial strains
The antimicrobial activity of lemongrass extracts was evaluated against Staphylococcus aureus, Escherichia coli, and Candida albicans using the agar well diffusion method. The ethanol extract from ultrasound-assisted extraction exhibited the largest inhibition zones, particularly against S. aureus (20.4 mm) and C. albicans (18.7 mm), indicating strong antibacterial and antifungal activities ([Table 4]).
|
Extraction Method |
Solvent |
S. aureus (mm) |
E. coli (mm) |
C. albicans (mm) |
|
Maceration |
Ethanol |
15.2 ± 1.3 |
12.4 ± 1.2 |
14.6 ± 1.2 |
|
Maceration |
Methanol |
14.8 ± 1.2 |
11.9 ± 1.1 |
13.9 ± 1.0 |
|
Soxhlet Extraction |
Ethanol |
18.3 ± 1.5 |
15.6 ± 1.4 |
16.8 ± 1.3 |
|
Soxhlet Extraction |
Methanol |
17.5 ± 1.4 |
14.7 ± 1.3 |
15.7 ± 1.1 |
|
Ultrasound-Assisted Extraction |
Ethanol |
20.4 ± 1.6 |
18.2 ± 1.5 |
18.7 ± 1.4 |
|
Microwave-Assisted Extraction |
Ethanol |
19.6 ± 1.5 |
17.3 ± 1.4 |
17.8 ± 1.3 |
|
Supercritical Fluid Extraction |
CO₂ |
13.5 ± 1.2 |
11.4 ± 1.1 |
12.8 ± 1.0 |
Discussion of the most effective extracts and possible ,odes of action
The ethanol extracts, particularly those obtained via ultrasound-assisted extraction, were the most effective against both bacterial and fungal strains. The high content of citral and other terpenoids likely contributes to the disruption of microbial cell membranes, leading to cell death.[6] The antimicrobial activity aligns with the high phenolic and flavonoid contents, which have been linked to microbial inhibition mechanisms such as oxidative damage and enzyme inhibition.[4]
Anti-inflammatory and cytotoxicity studies
Anti-inflammatory activity
The anti-inflammatory potential of lemongrass extracts was assessed via the inhibition of nitric oxide (NO) production in lipopolysaccharide (LPS)-stimulated macrophages. The ethanol extract from ultrasound-assisted extraction showed the highest inhibition of NO production (IC50: 28.3 µg/mL), outperforming methanol and water extracts, which exhibited lower activities.
Cytotoxicity studies
Cytotoxicity was evaluated using the MTT assay on human breast cancer (MCF-7) and normal fibroblast cells. The ethanol extract demonstrated selective cytotoxicity toward MCF-7 cells, with an IC50 value of 36.7 µg/mL, while showing no significant toxicity toward normal fibroblasts at concentrations below 100 µg/mL. This suggests the potential anticancer activity of the extract, likely due to the presence of bioactive compounds such as citral and geraniol.
Discussion
Extraction methods
Discussion on the efficiency of different extraction techniques
The extraction methods used in this study revealed varying efficiencies in terms of yield and phytochemical content. Ultrasound-assisted extraction (UAE) with ethanol achieved the highest yield and concentration of bioactive compounds, followed by Soxhlet extraction. UAE’s superior performance is attributed to the ultrasonic cavitation effect, which enhances the extraction efficiency by disrupting cell walls and facilitating solvent penetration.[10] Soxhlet extraction, while effective, required more time and solvent, which could be less sustainable for large-scale applications.[8] Supercritical fluid extraction (SFE), though eco-friendly, yielded lower amounts of bioactive compounds, possibly due to its limitations in extracting certain high-molecular-weight compounds.[12]
Analysis of how the choice of solvent and method influences phytochemical yield and bioactivity
The choice of solvent significantly impacted the extraction efficiency and phytochemical content. Ethanol was more effective than methanol and water, likely due to its intermediate polarity, which efficiently extracts a broad range of compounds, including both polar and non-polar substances.[9] The high phenolic and flavonoid contents in ethanol extracts contributed to their enhanced antioxidant and antimicrobial activities. In contrast, water, being a more polar solvent, extracted fewer non-polar compounds, resulting in lower overall bioactivity. [17]
Biological activity
Interpretation of antioxidant, antimicrobial, and anti-inflammatory activity results
The antioxidant activity of the lemongrass extracts, particularly those from UAE with ethanol, was the highest, demonstrating a strong scavenging effect on DPPH and ABTS radicals. This is consistent with the high phenolic and flavonoid contents observed, which are known for their strong antioxidant properties. The antimicrobial activity was also highest in ethanol extracts, indicating effective inhibition of microbial growth, likely due to the presence of compounds such as citral and geraniol that disrupt microbial cell membranes.[6] The anti-inflammatory activity, as evidenced by the inhibition of NO production, further supports the therapeutic potential of lemongrass extracts).[18]
Correlation between phytochemical content and biological activities
A strong correlation was observed between the phytochemical content (particularly total phenolics and flavonoids) and the biological activities of the extracts. Higher concentrations of these compounds corresponded to greater antioxidant and antimicrobial activities. This correlation aligns with previous studies, where phenolic compounds have been linked to potent antioxidant and antimicrobial properties.[19], [20] The presence of specific bioactive compounds such as citral was also associated with significant biological activities, reinforcing the importance of these compounds in lemongrass's therapeutic potential.[15]
Comparison with previous studies and literature
The findings of this study are consistent with existing literature, which highlights lemongrass as a potent source of bioactive compounds with significant antioxidant and antimicrobial properties.[17], [20] The high antioxidant and antimicrobial activities observed in this study align with reports from previous research, which attributed these effects to the presence of terpenoids and phenolics in lemongrass extracts (Sukhdev et al., 2008). The superior performance of UAE in this study also corroborates recent findings that emphasize the efficiency of modern extraction techniques over traditional methods.[10]
Potential Applications
Discussion on the therapeutic potential of lemongrass based on its biological activities
The demonstrated antioxidant, antimicrobial, and anti-inflammatory activities underscore the therapeutic potential of lemongrass. Its high phenolic content and bioactivity suggest that lemongrass extracts could be beneficial in developing natural antioxidants and antimicrobial agents, potentially contributing to the treatment of oxidative stress-related disorders and microbial infections.[18] The selective cytotoxicity observed in cancer cell lines further indicates potential for anticancer applications, making lemongrass a candidate for further research in cancer therapeutics.[21]
Possible uses in pharmaceutical, food, and cosmetic industries
In the pharmaceutical industry, lemongrass extracts could be utilized in formulations aimed at reducing oxidative stress and inflammation. In the food industry, they can be incorporated as natural preservatives due to their antimicrobial properties. Additionally, the cosmetic industry could benefit from lemongrass extracts in skincare products, where their antioxidant and anti-inflammatory properties could improve skin health and appearance.[6]
Suggestions for future research, including in vivo studies and clinical trials
Future research should focus on in vivo studies to evaluate the safety and efficacy of lemongrass extracts in live models. Clinical trials are also needed to establish the therapeutic benefits observed in vitro and in animal studies. Additionally, investigations into the synergistic effects of lemongrass extracts with other compounds could provide insights into enhanced therapeutic formulations.[18] Further studies could also explore the mechanism of action of the bioactive compounds to better understand their potential applications in medicine.
Conclusion
This study systematically investigated the extraction methods, phytochemical content, and biological activities of lemongrass (Cymbopogon citratus) extracts. The key findings are summarized as follows:
Most effective extraction methods and bioactive compounds
Ultrasound-assisted extraction (UAE) using ethanol was identified as the most effective method, yielding the highest amounts of bioactive compounds and demonstrating superior antioxidant, antimicrobial, and anti-inflammatory activities compared to other methods. This efficiency is attributed to the enhanced solvent penetration and disruption of cellular structures facilitated by ultrasonic waves. Among the bioactive compounds, citral emerged as the predominant component, contributing significantly to the observed biological activities.[15] Other notable compounds include geraniol and limonene, which also play crucial roles in the therapeutic potential of lemongrass.
Potential therapeutic applications of lemongrass extracts
The study highlights the considerable therapeutic potential of lemongrass extracts. The high antioxidant activity suggests potential use in preventing oxidative stress-related diseases. The antimicrobial properties indicate that lemongrass could serve as a natural preservative or therapeutic agent against microbial infections. Additionally, the anti-inflammatory and cytotoxic effects suggest applications in managing inflammatory conditions and potentially developing anticancer therapies . The versatility of lemongrass extracts makes them promising candidates for pharmaceutical, food, and cosmetic industries.
Importance of further studies
While the findings are promising, further research is necessary to validate the therapeutic potential of lemongrass extracts in clinical settings. In vivo studies and clinical trials are essential to confirm the safety and efficacy of lemongrass-based products. Additionally, exploring the synergistic effects of lemongrass extracts with other compounds could lead to enhanced therapeutic formulations. Future research should also focus on the development of standardized extraction protocols to ensure consistency and effectiveness in product development.
In conclusion, lemongrass demonstrates significant potential as a source of bioactive compounds with various therapeutic applications. The study provides a foundation for further exploration and development of lemongrass-based products, emphasizing the need for additional research to fully realize its benefits.
Source of Funding
None.
Conflict of Interest
There is no conflict of interest, The author alone is responsible for the content and writing of the paper.
References
- Shah G, Shri R, Panchal V, Sharma N, Singh B, Mann A. Scientific basis for the therapeutic use of Cymbopogon citratus, stapf (lemongrass). J Adv Pharm Technol Res. 2011;2(1):3-8. [Google Scholar]
- Mukhopadhyay M, Mukherjee A, Chaudhuri B. Lemongrass: A review of its medicinal applications. J Nat Remed. 2012;12(3):187-94. [Google Scholar]
- Blanco L, Marín C, Pérez R. Lemongrass (Cymbopogon citratus) essential oil: A review of its chemical composition, biological activities, and uses in the food, cosmetic, and pharmaceutical industries. J Essen Oil Res. 2020;32(4):1-10. [Google Scholar]
- Gbenou D. Antioxidant and antimicrobial activities of lemongrass (Cymbopogon citratus) extracts. J Med Plants Res. 2013;7(1):27-34. [Google Scholar]
- Sousa WP, Soares MP, Barros SD. Antioxidant properties and phytochemical screening of Cymbopogon citratus leaves. Int J Pharm Pharm Sci. 2012;4(4):122-6. [Google Scholar]
- Tyagi A, Malik A. Antimicrobial potential and mode of action of Cymbopogon citratus essential oil against Escherichia coli and Staphylococcus aureus. Asian Pacific J Trop Med. 2011;4(3):208-13. [Google Scholar]
- Mirghani M, Liyana A, Parveen J. Bioactivity analysis of Cymbopogon citratus essential oil. Int Food Res J. 2012;19(1):569-75. [Google Scholar]
- Kumar P, Mishra S, Malik A, Satya S. Insecticidal properties of Cymbopogon citratus essential oil and its efficacy against housefly (Musca domestica). J Esse Oil Bear Plants. 2012;15(3):466-71. [Google Scholar]
- Sukhdev S, Suman P, Gennaro L, Dev D. . Extraction technologies for medicinal and aromatic plants. 2008. [Google Scholar]
- Duarte F. Ultrasound-assisted extraction of essential oil from lemongrass (Cymbopogon citratus): Optimization and evaluation of antioxidant activity. J Med Plants Res. 2019;13(15):341-8. [Google Scholar]
- Hayat K, Zhang X, Chen H, Xia S, Jia C, Zhong F. Efficient extraction of polyphenols and antioxidants from fruit peels using microwave-assisted extraction. J Chromatog. 2010;1217(26):4511-8. [Google Scholar]
- Grosso C, Figueiredo A, Barroso J. Supercritical fluid extraction of essential oils: A short review. Flavour Fragrance J. 2012;27(6):401-6. [Google Scholar]
- Harborne J. . Phytochemical methods: A guide to modern techniques of plant analysis. 1998. [Google Scholar]
- Chang C, Yang M, Wen H, Chern J. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10(3):178-82. [Google Scholar]
- Adams R. . Identification of essential oil components by gas chromatography/mass spectrometry . 2007;456. [Google Scholar]
- Duarte S, Oliveira J, Lima E. Ultrasound-assisted extraction and antioxidant activity of bioactive compounds from lemongrass (Cymbopogon citratus). J Food Sci Technol. 2019;56(7):3050-8. [Google Scholar]
- Kalita S, Saikia J, Das B. Extraction of lemongrass oil: A comparative study of extraction using ethanol, methanol, and water. J Essen Oil Res. 2013;25(3):155-60. [Google Scholar]
- Sosa S. Anti-inflammatory activity of Cymbopogon citratus and its essential oil. Phytother Res. 2013;27(11):1682-8. [Google Scholar]
- Gbenou J, Ahounou J, Akakpo H, Laleye A, Yayi E, Kotchoni S. Phytochemical composition and antioxidant activity of lemongrass (Cymbopogon citratus) extracts. Food Chem. 2013;138(4):1704-9. [Google Scholar]
- Barbosa LA, Pereira U, Martinazzo A, Maltha C, Teixeira R, Melo E. Influence of drying air temperature on the content and chemical composition of the essential oil of Cymbopogon citratus (lemongrass). Food Chem. 2015;152(13):212-8. [Google Scholar]
- Sartippour M. Anticancer properties of Cymbopogon citratus: A review. J Med Food. 2014;17(5):646-52. [Google Scholar]
- Abstract
- Introduction
- Overview of lemongrass: Botanical classification, geographical distribution, and its significance in traditional and modern medicine
- Importance of lemongrass in pharmacology: Antioxidant, antimicrobial, and anti-inflammatory properties
- Objectives of the study
- Materials and Methods
- Plant material collection and preparation
- Description of the collection site and conditions for lemongrass cultivation or procurement
- Methods for drying, powdering, and storing plant material before extraction
- Extraction methods
- Solvent extraction
- Green extraction techniques
- Comparison of extraction efficiencies based on yield and phytochemical content
- Phytochemical analysis
- Results
- Extraction yield
- Presentation of extraction yields for different solvents and techniques
- Comparative discussion of the effectiveness of each method
- Phytochemical composition
- Results from qualitative and quantitative phytochemical analyses
- GC-MS/LC-MS Data highlighting major bioactive compounds
- Antioxidant activity
- Antimicrobial activity
- Antimicrobial activity against different microbial strains
- Discussion of the most effective extracts and possible ,odes of action
- Anti-inflammatory and cytotoxicity studies
- Discussion
- Extraction methods
- Discussion on the efficiency of different extraction techniques
- Analysis of how the choice of solvent and method influences phytochemical yield and bioactivity
- Biological activity
- Interpretation of antioxidant, antimicrobial, and anti-inflammatory activity results
- Correlation between phytochemical content and biological activities
- Comparison with previous studies and literature
- Potential Applications
- Conclusion
- Most effective extraction methods and bioactive compounds
- Potential therapeutic applications of lemongrass extracts
- Importance of further studies
- Source of Funding
- Conflict of Interest
- References
How to Cite This Article
Vancouver
Dwivedi M. Phytochemical characterization and biological evaluation of lemongrass (Cymbopogon citratus) extracts: A systematic experimental study [Internet]. Int J Pharm Chem Anal. 2024 [cited 2025 Oct 13];11(3):253-259. Available from: https://doi.org/10.18231/j.ijpca.2024.036
APA
Dwivedi, M. (2024). Phytochemical characterization and biological evaluation of lemongrass (Cymbopogon citratus) extracts: A systematic experimental study. Int J Pharm Chem Anal, 11(3), 253-259. https://doi.org/10.18231/j.ijpca.2024.036
MLA
Dwivedi, Mahendra. "Phytochemical characterization and biological evaluation of lemongrass (Cymbopogon citratus) extracts: A systematic experimental study." Int J Pharm Chem Anal, vol. 11, no. 3, 2024, pp. 253-259. https://doi.org/10.18231/j.ijpca.2024.036
Chicago
Dwivedi, M.. "Phytochemical characterization and biological evaluation of lemongrass (Cymbopogon citratus) extracts: A systematic experimental study." Int J Pharm Chem Anal 11, no. 3 (2024): 253-259. https://doi.org/10.18231/j.ijpca.2024.036
