Introduction
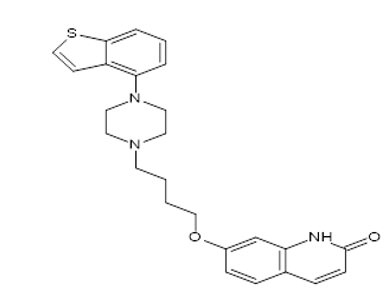
Brexpiprazole is a BCS class II type called (7-{4-[4-(1-benzothiophen-4-yl) piperazin-1-yl] butoxy} quinoline-2(1H)-one is a new medication in psychiatric1, 2 and also in the treatment of depression which has a high affinity for monoamine neurotransmitters like serotonin, dopamine, and noradrenaline receptors, For other major depressive disorder like Alzheimer’s disease, schizophrenia3, 4, neurobehavioral disorders5, combat disorder, bipolar disorder treatment, adjunctive treatment it is mostly used. It has more potency than other antipsychotic drugs with little aqueous solubility and substantial intestinal permeability.6, 7
Brexpiprazole was originally approved in the United States in July 2015 for use as an adjunctive action for major depressive disorder (MDD) and schizophrenia.9 Antipsychotics are first and second-generation drugs that are mainly active as D2 receptor antagonists.10, 11 Brexpiprazole acts as a partial agonist of the serotonin 5-HT1A receptor and the dopamine D2 and D3 receptors. Partial agonists have both blocking properties and stimulating properties at the receptor they bind to. The ratio of blocking activity to stimulating activity determines a portion of its clinical effects. .12 brexpiprazole is a substrate of CYP2D6 and CYP3A4, like its predecessor aripiprazole. Participants in the clinical trials are advised to avoid grapefruit, Seville oranges and related citruses. The literature survey discovered that by UV-visible spectroscopy and HPLC.13, 14, 15, 16, 17 method Brexpiprazole was determined. In the current work, the authors have proposed dissolution method development and validation of Brexpiprazole which is a simple, precise, robust, and valid RP-HPLC method for the estimation of Brexpiprazole in the pharmaceutical active dosage form.
Materials and Methods
Chemicals and solvents
Brexpiprazole standard (Purity ≥ 99.7), triethylamine, phosphoric acid, Methanol, HPLC grade water (Millipore).
Instrumentation
The instrument employed in the present work were analytical microbalance (Make: Mettler Toledo, Model: XP56), 1260 Infinity HPLC having column Hemochrom C-8, 25 cm X 4.6 mm x 5 µ, USP Apparatus Type II (Paddle), sonicator (Make: Elma, Model: S300H),
Method development
Optimization of the chromatographic conditions
The system used was Agilent 1260 Infinity HPLC having column Hemochrom, mobile phase was prepared by a combination of 20 ml of triethylamine with 1000 ml of HPLC grade water and 680 ml of HPLC Grade Methanol. pH was adjusted to 4.0 ± 0.05 with phosphoric acid, degassed by sonicator, and mix well. The diluent used was water. Analysis was carried out at wavelength 227 nm, flow rate 1ml per minute, 18-22 °C temperature, with an injection volume of 50 µl with time 21 minutes.
Preparation of working standards
The standard solution of Brexpiprazole prepared as 27.5 mg of pure drug was dissolved in a 250 mL volumetric flask and diluted to volume with water. Further 10 mL of stock was diluted to 50 mL with diluent, further, dilute 5 ml of the above-diluted solution to 10 ml with diluent.
Method validation
By analyzing linearity, precision (method and intermediate), accuracy, LOD, LOQ, deterioration, and robustness, the present created technique was verified according to ICH and FDA requirements.
Specificity
Specificity is the ability to access unequivocally the analyte in the presence of components that may be expected to be present. Separate vials were prepared and injected to check the interference. The standard solution was prepared to have a percentage purity of 99.61%. The analysis was done using various methods such as Blank solution, Control standard solution, Test solution and Placebo solution respectively.
Accuracy
Accuracy was conducted in the range of 20 % to 150 % of working concentration of 10 mg strength. Solutions of each accuracy level were prepared in triplicate. The study was performed by using placebo tablets of 10 mg strength. Determination of percent recovery was carried out in each study.
Linearity and range
The linearity parameter of an analytical procedure is its ability to obtain test results that are directly proportionally to the concentration of analyte in the sample. Linearity was conducted for the Brexpiprazole concentration between 20% to 150% level of limit concentration. Graphically region was plotted. The range was evaluated based on linearity.
Precision
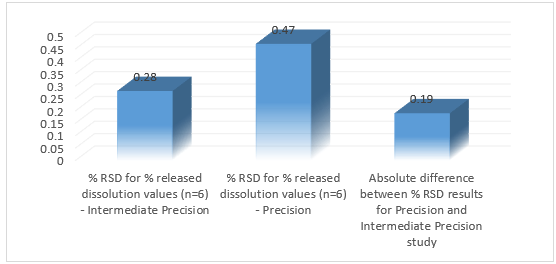
Precision was conducted by using tablets of 10.0 mg strength. This study was performed on 6 different jars. Standard solutions were prepared by purity 99.61%, Weight 27.69mg, and Concentration 11.03 µg/ml. In the precision determination method, the blank and standard solution method was used, also the intermediate precision determination method was done by using standard and blank solution.18
Robustness
The robustness was performed by using different samples with chromatogram injection and check the parameter to carrying out deliberate variations like Flow rate (±10%), Wavelength (+10 % at 230 nm and -10 % at 224 nm), RPM (±10%) and the volume of the dissolution medium (changed by ±10%)
Table 1
Method parameters of HPLC for robustness
Results
Method development
The developed chromatographic conditions were optimized to determine Brexpiprazole in drug substance and dosage form. The symmetrical peak was found with Hemochrom C-8, 25 cm X 4.6 mm x 5 µ or equivalent column at 18-22 °C, and the mobile phase consisted of 20 ml of Triethylamine with 1000 ml of HPLC grade water and 680 ml of HPLC Grade Methanol. Adjust the pH of this solution to 4.0 ± 0.05 with phosphoric acid. Detector performed at 227 nm and the flow rate was 1.0 mL/min. The injection volume was 50 µl and the run time was 25 min.
Specificity
The developed chromatographic method passed specificity criteria and the results of specificity are given the table 2-3.
Table 2
Summary of results for specificity study
Table 3
Result of specificity
Accuracy
The accuracy was done by analyzing samples at six different levels of concentrations the recovery of the method was determined by spiking Brexpiprazole active substances. The % recovery results are shown in table 4-5.
Table 4
Accuracy levels area response
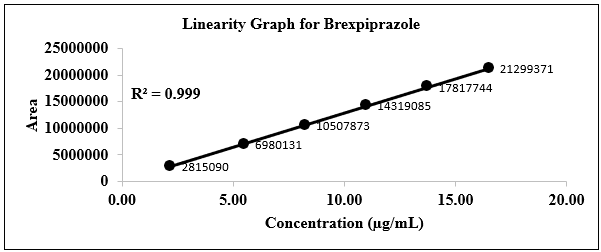
Linearity
Linearity was conducted for the Brexpiprazole concentration between 20% to 150% level of limit concentration. Range was evaluated based on linearity. Linearity summary is given in the graph is in figure 2 and in table from 6.
Table 6
Linearity and range results
Method Prescision
Table 7
Observation summary of test solution
Table 8
System suitability test for precision study
Table 9
Observation summary of test solution
Robustness
The robustness of the method was studied by small variation of the chromatographic conditions such as flow (1.0 ± 0.2 mL/min), column temperature (30 ± 5°C), and mobile phase composition (±10% absolute). The results are given in Table 10-16.
Table 10
Table 11
Parameters ofrobustness
Table 12
Observation summary of test solution for change in flow rate (+10%)
Table 13
Observation summary of test solution for change in flow rate (-10%)
Table 14
Observation summary of test solution for change in wavelength (+10%)
Table 15
Observation summary of test solution for change in flow rate (-10%)
Table 16
Observation summary of test solution for change in RPM (+10%)
Change in Rpm – Dissolution Apparatus
Table 17
Observation summary of test solution for change in volume of dissolution medium (+10%)
Table 18
Observation summary of test solution for change in volume of dissolution medium (-10%)
Discussion
In this RP-HPLC method, the Retention time of the Brexpiprazole peak in the test solution is comparable to that in the standard solution. It was found that interference of diluent and placebo is not more than 0.5% of Brexpiprazole. The individual % recovery was found to be between 97.0% to 100.0%, the linearity was within the range of 10- 60 µg mL with a Correlation Coefficient of 0.999, Slope of the regression line was found to be 1299178.9578 which means all parameters were found to be within range. In precision % RSD for % released dissolution values were found to be 99.16, in robustness, all the parameters like change in the flow rate, wavelength, and in RPM shows that the developed method was robust.
Conclusion
This study is a stability-indicating analysis that was done in accordance with ICH/FDA criteria. The proposed approach performed well in terms of sensitivity, precision, accuracy, linearity and range, robustness. The well-known RP-HPLC method for the study of Brexpiprazole was shown to be trustworthy, as well as easy, consistent, cost-effective, and exact. For quality control or routine quantification, this method is applicable to determine Brexpiprazole in a bulk and pharmaceutical dosage form. This developed method required less time.
Abbreviations
RP-HPLC- Reversed-phase high-performance liquid chromatography, USP- United States Pharmacopeia, LOD- Limit of detection LOQ- Limit of quantification, D2- Dopamine receptor, ICH- International Council on Harmonisation, FDA- Food drug administration, RPM-Rotation per minute, ND- Not detected, NA- Not applicable, RSD- Relative standard deviation.