- Visibility 23 Views
- Downloads 3 Downloads
- DOI 10.18231/j.ijpca.2024.034
-
CrossMark
- Citation
Formulation, development and evaluation of fast disintegrating tablet of dapsone by using natural super disintegrates
- Author Details:
-
Nandhini M *
-
Suganya T Kumar
-
Yamuna R
-
Keerthika A
-
Sindhu C
-
Voleti Vijaya Kumar
-
Anisha B
-
P. Shanmugapandiyan
Introduction
Oral Administration of the tabletsis the advantageous dosage form for because of many factors like its convenience in term of self-administration, accurate dosage and ease in manufacturing. Swallowing by the pediatric and geriatric patients is the limitation for the oral tablet dosage forms. The quick disintegration of the tablet in the oral cavity facilitates the quick drug release and quick onset of action. For this reason, the formulation of the Fast-disintegrating tablets plays a major role, which can be prepared by the use of the super disintegrating agents. The drug product dissolving in oral cavity with in few seconds of the time when it comes in contact with the salivary fluid without the consumption of the additional water. [1] The advantage of FDT is high patient acceptance, compliance and increase the oral bioavailability. Due to abundant availability and less interactions natural super disintegrating agents are advantageous over the synthetic super disintegrating agents. Dapsone is selected asAPI to formulate the FDTs. Dapsone is Class-II drug, shows less bioavailability. For this reason, Dapsone solid dispersions were prepared by the use of the solvent evaporation method.In this work, Banana powder, Plantago Ovata and Fenugreek Seed Mucilage were selected as natural super disintegrating agents. The tablets were prepared by the direct compression method.[2] Three super disintegrating agents were used in different proportions. The objective the current work is to improve the bioavailability of the dapsone by improving its solubility. The powder blend was subjected for the pre and post compressional parameters. Fenugreek gum and banana powder act by swelling mechanisms, which are responsible for drug release. Thus, the Three super disintegrating agents were comparedto find out which gives the best results.
Materials and Methods
The API Dapsone was procured as a gift sample from Chennai. Fenugreek seeds, Plantago Ovata and Banana powder was procured from the local market in Chennai. All the other chemicals and reagents used in the research work are of analytical grade and procured from SD fine chemicals, Mumbai.
Methodology
Physicochemical Characterization of Natural Super Disintegrants
Micrometric properties: The purified and dried extracted powders were evaluated for its organoleptic characteristics like colour, odour, taste, taste, shape, texture by visual and Microscopical observations.[3]
Swelling index: The study was carried out by usinga100mL stop Pere graduated cylinder. The initial bulk volume of 1 g of Powder was noted. Water was added in sufficient quantity to ensure 25 mL of uniform dispersion by vigorously shaking every 10 min for 1 h and then allowed to stand for 24 h. The dispersion was stored at room temperature and the sediment volume of the swollen mass was measured after 24 hours.
Swellingindex=100*(V2-V1/V1)
Where, V1=Initial volume of material before hydration;
V2=Volume of hydrated material
Determination of total ash: 1g of powder accurately weighed, in a suitable tared dish. Incinerate the material by gradually increasing the heat, not exceeding 450°C, until free from carbon, cooled, and weighed.[4] Total ash content can be calculated from
%ashcontent=c-a/b-a×100
Weight of emptysilicacrucible=(a),
Weigh tof crucible with 3g of sample=(b),and
Weight of crucible with ash = (c)
Lossondrying: The loss on drying technique is used to determine high levels ofmoisture orsolvents present in the sample. The material sample was weighed(W1)and heate dinanoven for 2 h. It was cooled in the dry atmosphere of desiccators and then finally weighed (W2).
% Lossondrying=[(W1-W2)/W1]100
Where, W1=Initial weight of the powder; W2= Final weight of the powder
Moisturecontent: The moisture content was then determined as the ratio of weight of moisture loss to weight of sample expressed as a percentage
% Moisture Content=b-c/b-a×100
Weight of weighing bottle,
Weight of weighing bottle with1gof sample,
Weigh to fweighingbottlewithsampleafterdryingat105°C for 2h
Preformulating studies
Melting Point: The melting point of the drug substances was determined by using melting point apparatus (PMP-D, Veego). The melting point was determined by introducing small amount of substance in the capillary attached to graduated thermometer and constant heat was applied with the assembly suspended in the paraffin bath. The drug sample was tested in temperature range 100-2500C and point at which drug melts was noted. The melting point is reported in section.
Solubility: Solubility of the Dapsone was determined in different solvents like water, 0.1 N HCl, phosphate buffer pH 6.4, alcohol, acetone etc.
UV absorption maxima of Dapsone: UV scanning was done in Shimadzu double beam UV/Visible spectrophotometer using 10 µg/ml drug solutions in the wave length range of (200-400 nm). Phosphate buffer 6.4 used as a blank.
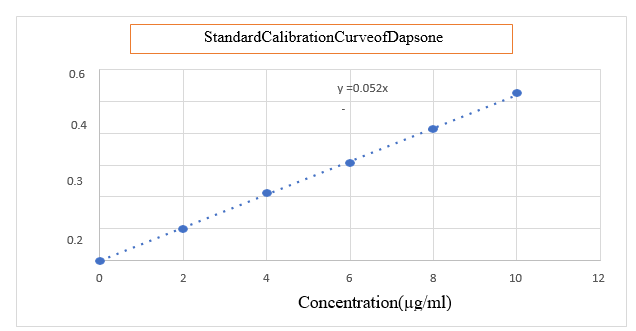
Preparation of Standard Curve: From the stock solution 2,4,6,8 and10 ml were transferred to10ml standard volumetric flasks and diluted with phosphate buffer the (PH6.4) upto themark to obtain Dapsone concentration of 2, 4, 6, 8and 10 µg/mlrespectively. Absorbanceofeachsolutionwas measured at 260 nm.
Preparation of Solid Dispersion: Solid dispersions of Dapsone were prepared by solventevap oration method. Accurate amount of dapsone was weighe dandtakenina Chinadish, dissolve dinmethanol and then carrier was added (Urea in ratio of 1:1, 1:2 &1:3). The solventwas evaporated at room temperature and dried in hot air oven at 500C for 4 hours. The resultant mass was passed through sieve no. 60 and stored in desiccator. [4], [5]
Characterization of solid dispersions
Drug content: An accurately weighed quantity of solid dispersion equivalent to 25mg Dapsone was taken into 100ml of volumetric flask. Dissolved in phosphate buffer pH 6.4 and the volume were made up with the same. An aliquot of the filtrate was diluted and analyzed spectro photometrically (UV-1700, Shimadzu Corporation) at 260 nm.
Dissolution study
Invitro dissolution studies of Dapsone in powder form, Dapsone Solid Dispersions (DSD) and Dapsone Physical Mixture (DPMs) were performed by using the USPXXIII type-II dissolution apparatus (Electrolab TDT-06N) employing a paddle stirrer at 50 rpm.[6]
900 ml of pH 6.4 phosphate buffer was used as dissolution medium. The temperature of dissolution medium was maintained at 37 ± 0.5oC throughout the experiment. Samples of dissolution medium(5ml) were with drawn for 20 min by means of syringe fitted withpre-filterat known intervals of time and analyzed for drug release by measuring the absorbance at 260 nm. The volume withdrawn at each time interval was replaced with fresh quantity of dissolution medium. Cumulative percent released was calculated and plotted against time.[7]
Formulation of Dapsone FDT by use of Natural Super Disintegrants
Fast dissolving tablet of Dapsone were prepared by direct compression method. Pure drug and natural super disintegrants were passed through 60 No. mesh, required amount of drug and excipients were taken for every formulation. The powdered drugand excipients were mixed uniformly with continuous trituration using mortar and pestle. Then weighed quantity of natural super disintegrants properly mixed, finally magnesium stearate and talc powder were added and mixed well. The mixed blend of drug and excipients were compressed using 10 station tablet punching machine.Before the tablet compression the mixture blend of all designed formulations were subjected topre-compression parameters like- Angle of repose, Bulk density, Tapped density, compressibility index, Hauser’s ratio.
Evaluation of the Prepared Tablets
All prepared tablets of Meclofenamate Sodium were evaluated for the following parameters as per IP guideline.
Weight Variation: Twenty tablets were selected at random and averageweight was determined. Then individual tablets were weighed and the individual weight was compared with an average weight.
Content uniformity test: Ten tablets were weighed and powdered, a quantity of powder equivalent to 50 mg of Dapsone was transferred toa25ml volume tricflask and15ml wateris added. The drug is extracted in water by vigorously shaking the stoppered flaskfor15 minutes. Then the volume is .032 adjusted to the mark with distilled water and the liquid is filtered. The Dapsone content was determined by measuring the absorbance at 260 nm after appropriate dilution. The drug content was calculated using the standard calibration curve. The mean percent drug content was calculated as an average of three determinations.
Disintegration test: Tablets were taken and introduced in each tube of disintegration apparatus, and the tablet rack of the disintegration apparatus was positioned into a 1-liter beaker containing 900 ml of distilled water and the time of disintegration was recorded. To discriminate between the formulation’s disintegration was doneat room temperature and disk was not used for the study.[8]
In vitro dispersion time: Tablet was added to 10 ml of pH 6.4 phosphate buffer solution at 37 ± 0.5oC. Time required for complete dispersion of a tablet was measured.
Wetting time and Water absorptionratio: A piece of tissue paper folded twice was placed in a small petridish (internal diameter 5 cm) containing 6 ml of water. A tablet was put on the paper and the time required for complete wetting was measured, the wetted tablet was then weighed.
Dissolutionstudy: In vitro dissolution of Dapsone mouth dissolving tabletswas studied in USP XXIII type- II dissolution apparatus (Electrolab TDT-06N)employing a paddle stirrerat 50 rpm. 900 ml of pH 6.4 phosphate buffer was used as dissolution medium. The temperature of dissolution medium was maintaine dat37±0.5o Cthroughoutt he experiment. One tablet was used in eacht est. Samples of dissolution medium (5ml) were with drawn by means of syringe fitted withpre-filter at known intervals of time and analyzed for drug release by measuring the absorbance at 260 nm The volume withdrawn at each time interval was replaced with fresh quantity of dissolution.[9], [10]
Results
|
S. No |
Physical Property |
FenugreekSeed Mucilage |
Bananapowder |
Plantago Ovata |
|
1 |
Colour |
White |
White |
LightBrownish |
|
2 |
Odour |
Odourless |
Odourless |
Odourless |
|
3 |
Taste |
Tasteless |
Tasteless |
Tasteless |
|
4 |
Shape |
Irregular |
Irregular |
Irregular |
|
5 |
Texture |
Soft |
Soft |
Soft |
|
S. No |
Parameter |
Fenugreek Seed Mucilage |
Banana powder |
Plantago Ovata |
|
1 |
% Swelling Index |
68±0.27 |
55±0.27 |
74±0.27 |
|
2 |
% Losson |
1.81±0.27 |
1.55±0.27 |
0.74±0.27 |
|
3 |
% Moisture Content |
3.81±0.14 |
2.15±1.11 |
2.74±0.23 |
|
4 |
Total |
2.61% |
3.15% |
2.14% |
|
S. No |
Formulation |
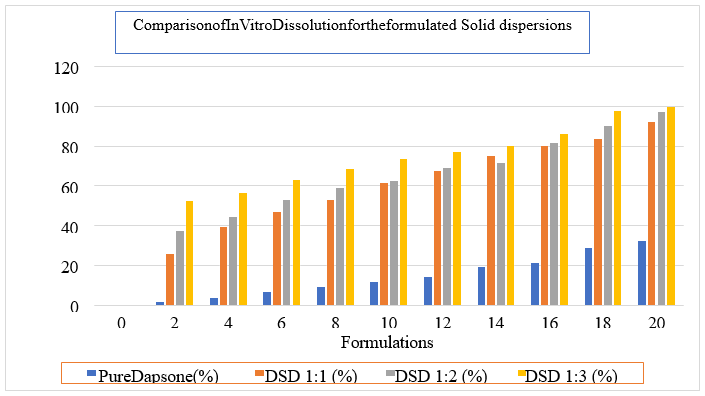
Cumulative % drug release after 20 min. |
|
1 |
DRUG |
32.16±2.45% |
|
2 |
DPM1:1 |
39.14±1.05% |
|
3 |
DPM1:2 |
51.18±1.27% |
|
4 |
DPM1:3 |
62.18±2.11% |
|
5 |
DSD1:1 |
92.16±1.34% |
|
6 |
DSD1:2 |
97.22±1.24% |
|
7 |
DSD1:3 |
***99.62±1.02% |
|
Formulation code |
Bulk Density (g/cc) |
Tapped density (g/cc) |
Angleof repose (degree) |
Carr’s index (%) |
Hausner’s ratio |
|
F1 |
0.47 |
0.57 |
25.40 |
14.04 |
1.14 |
|
F2 |
0.43 |
0.56 |
25.06 |
12.72 |
1.16 |
|
F3 |
0.45 |
0.57 |
24.38 |
13.20 |
1.14 |
|
F4 |
0.42 |
0.49 |
25.72 |
12.24 |
1.15 |
|
F5 |
0.42 |
0.43 |
25.94 |
12.76 |
1.13 |
|
F6 |
0.38 |
0.42 |
25.48 |
11.36 |
1.12 |
|
F7 |
0.43 |
0.66 |
26.21 |
14.06 |
1.12 |
|
F8 |
0.52 |
0.62 |
25.74 |
13.11 |
1.15 |
|
F9 |
0.46 |
0.56 |
23.02 |
13.79 |
1.14 |
|
Formulation Code |
Hardnes s (kg/cm2) |
% Friability |
In vitro Dispersion Time (Sec) |
In vitro Disintegration Time (Sec) |
% Drug Content |
Weight variation |
|
F1 |
2.93 |
0.33 |
30.15 |
32 |
88.26 |
246.81 |
|
F2 |
2.76 |
0.32 |
32.23 |
30 |
84.02 |
248.72 |
|
F3 |
2.91 |
0.35 |
31.55 |
29 |
85.45 |
248.81 |
|
F4 |
2.88 |
0.36 |
31.23 |
28 |
91.94 |
249.83 |
|
F5 |
2.46 |
0.37 |
32.15 |
28 |
89.84 |
248.15 |
|
F6 |
2.35 |
0.32 |
27.12 |
25 |
91.41 |
246.45 |
|
F7 |
2.35 |
0.35 |
23.14 |
25 |
93.41 |
249.81 |
|
F8 |
2.37 |
0.36 |
23.14 |
23 |
96.41 |
249.73 |
|
F9 |
2.61 |
0.32 |
21.15 |
20 |
98.41 |
248.82 |
Discussions
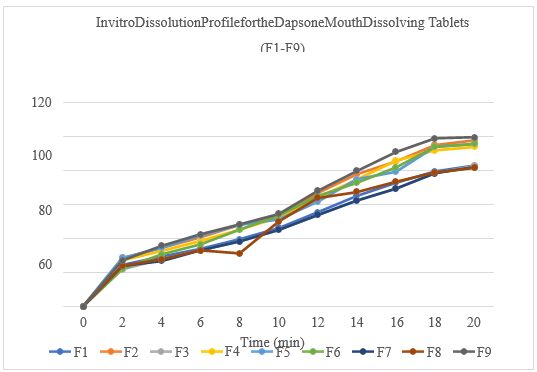
All the Natural super disintegrant’s extracted from the natural sources characterized by their morphological characteristics and it was observed that all the super disintegrant’s showed the good results. The swelling index was found to be more for the Plantago ovata i.e 74 % which may indicative of the more porosity, which is required for the drug release.The moisture content, Loss on drying and Total Ash was performed for the natural excipients, all the values are within the limits as per the standards. The purity also characterized by the determination of the melting point by the capillary tube method the value is within the limits. The solubility of the dapsone was done by use of different olvents, it provesthat, dapsone showed poor solubility in the water and soluble in some organic solvents. Base dothiepin, it was decided to improve the solubility of the dapsone by preparing the solid dispersions with the help of urea by solvent evaporation method. The formulated solid dispersions characterized for the drug content, dissolution, Xray diffractions and FTIR studies. The solid dispersions prepared by the solvent evaporation with the ratio of1:3 showed better drug content, dissolution (99.62 %) at the 20 min. The angle of repose for the entire formulations blend was found to be in therange 23.02oto 26.21 o. Hausner ratio was found to be in the range 1.12 to 1.16and that indicated that all formulation has good flow properties. Carr’s index was found to be in the range 11.36% to 14.06% that indicated all formulation has good flow properties. Allt he formulated(F1toF9) tablets were passed weight variationtestas the% weight variation was within the IP limits. The maximum friability of the formulation was found to be 0.37%. The minimum friability of the formulation was found to be 0.32%. The % friability was less than 1% in all the formulations ensuring that the tablets were mechanically stable. The maximum drug content for the all formulation was found to be 98.41% and minimum% drug content from the all formulation was found to be 84.02%. The results were within the limit specified by the IP. In vitro Disintegration time was found to be in the range 20 to 32 sec. From all formulations, F9 (Banana Powder) has minimum disintegration time 20.sec.Dissolution data shows that formulation F9 having (Banana Powder) gave improved dissolution as compared to other formulations and total drug release of 99.43% was found at 20 min.
Source of Funding
None.
Conflict of Interest
None.
References
- CA Lipinski, F Lombardo, BW Dominy, PJ Feeney. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 2012. [Google Scholar]
- V Dave, RB Yadav, R Ahuja, S Yadav. Formulation design and optimization of novel fast dissolving tablet of chlorpheniramine maleate by using lyophilization techniques Bulletin of Faculty of Pharmacy. Bull Faculty Pharm Cairo Univ 2017. [Google Scholar]
- S Kumar, SKR Garg. Fast Dissolving Tablets: Current Status, New Market Opportunities, Recent Advances In Manufacturing Technologies And Future Prospects. Int J Pharm Pharm Sci 2014. [Google Scholar]
- P Malik, RK Malik, N Gulati, U Nagaich, B Rastogi. Recent patents on fast dissolving tablets - A Review. Asian J Pharm Life Sci 2012. [Google Scholar]
- H Prasad, NK Verma. A Review On Patent Related Technologies Of Orally Disintegrating Tablets. World J Pharm Res 2014. [Google Scholar]
- D Sharma. . Formulation development and evaluation of fast disintegrating tablets of Salbutamol Sulphate for Respiratory Disorders 2013. [Google Scholar]
- S Kumar, TN Satya Yagnesh. Optimization and Evaluation of Aceclofenac Fast Dissolving Tablets Employing Starch Xanthate-A New Superdisintegrant. Int J Chem Tech Res 2017. [Google Scholar]
- D Shukla, S Chakraborty, S Singh, B Mishra. Mouth Dissolving Tablets II: An Overview of Evaluation Techniques. Sci Pharma 2009. [Google Scholar]
- S Kumar. Optimization and Evaluation of Aceclofenac Fast Dissolving Tablets Employing Starch Xanthate-A New Superdisintegrant. Int J Chem Tech Res 2017. [Google Scholar]
- VK Voleti, SP Bolla. Formulation and evaluation of levociterizine dihydro chloride fast dissolving tablets using superdisintegrants. Int J Pharm Pharm Sci 2012. [Google Scholar]
