Introduction
The contagion had a devastating influence on the entire planet in this current uncertain life-changing circumstance caused by the crisis of SARSCoV-2, or the severe acute respiratory syndrome coronavirus. 1 Family Coronaviridae is a single chain with a good recognise RNA genomes that are 25-32 kb in length and include spiky elongation visible under an electron microscope. 2 As per the conclusion blood-brain barrier (BBB) is crossed by SARS-CoV-2 and infects neurons and glial cells which act as ACE2 expressers and also cause neuronal inflammation and pathogenesis. The brain portion like the hypothalamus controls numerous physiological functions. i.e. body temperature regulation and hormonal balance. Although the fact that SARS-CoV-2's primary infective targets are the lungs, mounting research points to concurrent clinical findings, including infection of the testicles and brain. and multiorgan anomalies in patients with COVID-19. 1 Prior research has shown that the SARS-CoV-2 entrance into an individual's body is caused by the angiotensin-converting enzyme 2 (ACE2) receptor. Further, this attaches to virus protein and active surface transmembrane protease serine 2 (TMPRSS2), which allows the fusion of virus receptors. The pulmonary, cardiovascular, renal, gastrointestinal tract and urogenital systems are various human tissue that consists of ACE2 which make them sensitive to the virus.3 For COVID-19 pathophysiology, the Angiotensin Convert Enzymes 2 (ACE2) receptor is essential because it aids in the direct death of host cells. Cells that express more ACE2 are more prone to SAR-CoV2 infection. Four major testicular cell types such as spermatogonia, Leydig cells, Seminiferous duct cells, and Sertoli cells express ACE2 mRNA.4
Several research implies that the 2019 coronavirus disease has had an impression on male infertility Since December 2019, the illness has expanded globally, resulting in severe acute respiratory syndrome, which has been dubbed by the World Health Organisation (WHO) (2020), and WHO (Organisation, 2020) has classified it as a global outbreak.5 As previously documented, the first stage in SARS-CoV-2 infection is the fusion of its spicule proteins with host cell receptors by proteases, primarily the transmembrane protease serine 2 (TMPRSS2). It has been proposed that TMPRSS2 cleaves the ACE2 receptor, facilitating viral entrance into host cells. Human studies show activation of TMPRSS2 gene transcription with the activation of androgen receptors is needed. As a result of the presence of the androgen receptor and ACE2, which are gene locations on chromosome X, which facilitate the X-linked transmission through inherited variants, male vulnerability to COVID-19 is explained. and, as a result, explains male susceptibility to COVID-19. Which contain endogenous androgen activities may be a plausible mechanism. ACE2 expression in the testis has been linked to age. The highest levels of expression have been recorded in patients having 30 aged, while those aged 60 have the lowest levels of expression. COVID-19, which is an acute severe inflammatory disease, may decrease activation of the hypothalamic-pituitary-testicular (HPT) axis, resulting in stubby follicle-stimulating hormone (FSH), and testosterone level and luteinizing hormone (LH). A study regarding COVID-19 male patients found lower serum testosterone levels, greater LH levels, and a poorer T: LH ratio when differentiated from age-matched controls.4 Several scientific studies have been conducted on diverse symptoms and multisystem failure with the exception of the genital system which is a less studied system that has the ACE2 receptor. Because reproductive health is a growing concern between populations and its negative consequences can accelerate various complications ranging from physical disruption to psychological difficulties. Previous studies attempted to pose a significant claim to SARS-CoV-2 having short- and everlasting-term genital complications and depict due to virus-receptor binding activity where it causes direct damage to the male reproductive system containing potential target.3 Hence, male fertility has become a hot field among researchers Because there is a scarcity of evidence on the consequence of COVID-19.2
Comorbidities and their effect on SARS-CoV19
The hazard ratio (HR) for in-hospital fatality in 191 COVID-19 patients was 3.05, while the HR for death and noncardiogenic pulmonary edema in 201 COVID-19 patients was 1.70-1.82. Similarly, cardiovascular comorbidities are linked to an increased risk of death.6 Considering that patients with serious illnesses continue to perform worse than those who were immunized without any of the examined comorbidities in terms of outbreak and infection and consequent hospitalization rates, our data justify the use of booster immunizations for these individuals. Supplemental immunizations will most likely assist those with comorbidities, such as those with chronic renal disease, in order to strengthen and increase their defensive response to disease.7 Age, gender, comorbidities, chronic medications, the length of time between the onset of symptoms and ED admission, the types of medication strategies administered while in the ED (hydroxychloroquine, antiviral, antibacterial, Lower molecular mass heparin (LWMH) treatment), the number of days spent in the hospital, and the prognosis in terms of survival were all data that were gathered. The data were kept on a local server, accessible only to those with permission, and restricted for this study. 6 After controlling for age and other demographic characteristics, we found long-term kidney illness as the more risk with individual comorbidity in hospitalization. This finding is consistent with prior research that looked at disparities During the COVID-19 outbreak, rates of infection were high in the United Kingdom.
Prior research on unvaccinated and vaccinated populations has found that people with highly unsafe comorbidities particularly as lung disease, Diabetes, chronic kidney disease (CKD), and hypertension, or who are having a weak immune system (For instance, due to cancer, a transplanted organ, etc.) have a worse outbreak for COVID-19 infection than humans without those conditions. Furthermore, crystal clear interlinkage among comorbidities has not always been examined as earlier analysis has tended to concentrate on a couple of inherently connected comorbidities or on comprehensive analyses that anchor on incidence rates of hospitalization. We selected to investigate these comorbidities because of their frequency in US citizens, their relationship with reduced innate immune function, and recent research revealing risk variables linked associated with COVID-19 hospitalization in both vaccinated as well as unvaccinated individuals. We postulated that patients who had a mix of those four comorbidities may experience an interaction effect that affects the chance of hospitalisation following a breakthrough infection, similar to our analysis of the time it took to develop the infection, similar to our analysis of the time it took to develop infection. After an innovative infection, we developed four hospitalization models, each with a different degree of interaction. The fundamental model makes the assumption that co-morbid conditions have just an additive impact and that having several co-morbid conditions carries no additional risk. patients who were newly identified with one of the criticized forms of comorbidities were excluded from the study, after administration of their initial dose sequence within 14 days.
For each comorbidity that has no interaction factors, the hazard ratio is typically calculated using the mounting regression coefficients from the COX regression framework. We used the means R program to calculate the risk ratios comparing a patient with more than one comorbidity to a patient lacking comorbidities in their medical history. patients consisting of two or more of the comorbidities have an even higher chance would be expected based on unconstrained effects of comorbidities. furthermore discovered that patients with any one of the four comorbidities with an elevated chance of COVID-19 infection differentiate beyond any complications. 7
The molecular mechanism associated with low sperm count
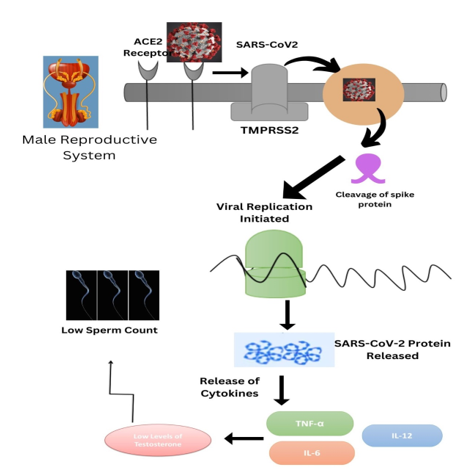
The theorized framework of SARS-CoV-2 entrance associated interaction of Spike protein (S-protein) infectious activator of SARS-CoV-2 to the organized ACE2 receptor. The serine proteolytic co-receptor TMPRSS2 then breaks down the Spike protein, allowing the membranes of the virus and cell to unite. The testes show an abundance of ACE2 receptor expression on fertilized eggs, seminal tubules, Leydig cells, and Sertoli, among other areas. Males who were infertile and had serious spermatogenesis abnormalities showed a lower level of ACE2 than those who were fertile. 8 Proteins are well-called SARS-CoV-2 entrance factors. ACE-2, TMPRSS2 and CD147 receptors have been found located in the follicles of the ovary, testicles, epididymis, prostrate, and yolk sacs. according to research. The RAS modulates tubular contractility, sperm development, male reproductive cell development, acrosomal exocytosis, sperm stimulation, and fertilization are abilities in males. 9
HIV, Mumps, and Zika, which are (single-stranded RNA viruses) including coronavirus types have been linked to groin pain and can be identified in sperm. A variety of cells co-express both ACE2 and TMPRSS2 at various quantities. Co-expression's absence would suggest a low likelihood of immediate invasion or a different pathway of the entrance. The amount of ACE2 mRNA (messenger RNA) and TMPRSS2 in the testis was measured during an autopsy. 5 people having COVID-19 were studied, and it was shown that every individual expressed more ACE2 and TMPRSS2 than controls pursued in their seminiferous tubules. The likelihood of SARS-CoV-2 promptly infiltrating and harming the testicles is raised by elevated activation. Uncertainty exists regarding the cause of the elevated levels of ACE2 and TMPRSS2. 8 The degree of the illness Interleukins (for example, (such as IL-1, IL-2, IL-7, and IL6), the tumor necrosis factor (TNF), interferon, which stimulated protein 10 (IP-10), monocyte-specific chemoattractant protein-1 (MCP-1), granulocyte-colony promoting factor (GCPF), and macrophages irritation protein-1 (MIP-1) were among the inflammatory chemicals with greater expression in COVID-19 patients. 10
It has been discovered that the regulation of ACE2 in mouse Leyding cells, human Leydig, and Sertoli cell lines is crucial for controlling the function of sperm cells, especially Leydig cells, which are thought to have an impact on steroid production and other activities. On the other hand, the presence of the angiotensin-2 receptor present on the Leydig cell has been found. In Leydig cells, Ang II inhibits either fundamental and LH-stimulated production of testosterone. The results of the TMPRSS2 and ACE2 tests were confirmed. In addition, histological analyses revealed the presence of several white corpuscles (such as HLA-DR b-cells, CD68 scavenger cells, CD3 T cells, CD20 B cells, and blood cells). Through the ACE2 receptor, ARS-CoV-2 infects target cells, as previously described. As a result, The potential for regional infection in the intended organ is increased by the existence of ACE2 on the cell pavement. Inflammatory mediator levels (including TGF-, IL-10, IL-8, IL-6, IL-1, IFN- and TNF-) are dysregulated. 10
ACE2 is an aspect of the RAAS
The equilibrium of the body is managed by the Renin-Angiotensin-Aldosterone System (RAAS), the hormonal cycle that governs the pressure in the arteries and external to cell volume. angiotensinogen to angiotensin I is translated by Renin and this enzyme activated the RAAS system. The remaining aspect of the pathway is mainly generated by the various responsibilities that the angiotensin-converting enzyme (ACE) and ACE2 mediate. The C-terminal dipeptide translates angiotensin I (Ang I) to angiotensin II (Ang II) which is eliminated by the embedded membrane exopeptidase known as ACE. ACE2 has two domains, like ACE, an extracellular enzymatic domain and a proximal end. ACE2's enzymatic region contains 42% of its characteristics along with ACE. The gene located on chromosome Xp22 transcripts for ACE2, an 80S amino acid type I membrane-based protein. 11
Semen Functionality and prostate association in the case of COVID‐19
The essential issue as previously said, a tiny proportion of prostatic elevation and clumped cells indicate ACE 2, whereas the human prostate membrane strongly expresses TMPRSS2; thus, it is more likely to become infected with SARSCoV2, these processes could explain the study's SARSCoV2 (+) sperm samples. It is additionally recognised that at the time of ejaculation, the muscles of the prostate gland help push the seminal liquid into the urinary bladder. Prostate fluid, among the main pivotal elements, is secreted by the prostate region. Notably, the central nervous system is essential for endocrine regulation and sperm development. The Hypothalamic Pituitary Gonadal Axis (HPGA) is important in the reproductive process since it can suppress the body's reproductive activities through hormones. Furthermore, HPGA stimulation and resultant modifications in hormone levels play an important impact in the low integrity of sperm. The brain's GnRH-revealing interneurons release GnRH, which stimulates The luteinizing hormone (LH) and follicle-stimulating hormone, or FSH, to be released by the pituitary gland on the anterior side. GnRH deficiency reduces FSH and LH levels, impairing the functionality of the Sertoli and Leydig cells. COVID-19 patients had considerably greater serum LH levels than healthy men, but lower testosterone/LH and FSH values, indicating possible hypogonadism. Taken as a whole, COVID-19 patients had a lower testosterone/LH ratio, reflecting potential preclinical dysfunction of the male gonadal system. 3
Causes of male infertility in SARS-CoV-2 patient
It was discovered that SARS-CoV-2 changed the hormonal components necessary for maintaining the health of reproductive function of male in COVID-19 patients. 1 The SARS-CoV-2 virus has the capability to infect male reproductive parts. 2 The cardiac system, gastric system, kidneys, brain tissues, and testes all have high levels of ACE2 receptor expression. With a stronger affinity than SARS-CoV-1, SARS-CoV-2 may enter host cells through this receptor. 2 The action of SARS-CoV-2 to use the cellular receptor ACE2 as a pathway to the male reproductive system. In testes, ACE2 is extensively expressed in spermatogonia, Leydig, and cells of Sertoli. Additionally, it is speculated that SARS-CoV-2 attachment to the ACE2 receptor may boost ACE2 expression and trigger an inflammatory response, which may interfere with Sertoli and Leydig cells' regular operations.1 The potential for male reproductive tissues to become infected by SARS-CoV-2 due to the occurrence of ACE2 receptors.3 ACE2 receptors are important in the pathophysiology of COVID-19, and the virus may target and harm cells that express ACE2 at high levels.4 The expression of ACE2 in male and female gonads differs, which is another possible explanation for the disparity in SARS-CoV-2 prevalence and severity between male and female patients. Long-term angiotensin II exposure can also result in early acrosomal exocytosis followed by sperm sense initiation because angiotensin II enhances the acrosome reaction in human sperm. Seminiferous tubules exhibited inflammatory infiltration, and immunohistochemical examination revealed deposition of IgG in the Sertoli cells, interstitium, degenerating germ cells, and seminiferous epithelium, indicating testicular injury caused by the testes' immune response. 5 Infection with SARS-CoV2 has been linked to decreased sperm quality, severe damage to the tubules of seminiferous tissues, a decline in Leydig cells, and mild lymphocytic inflammation. 6 Although male gamete production was reduced, SARS-CoV-2 infection caused ACE2-positive cells to become infected, and infected ACE2-positive spermatogonia are substantially enriched in viral reproduction and transmission. 5 Testicular damage may also occur in COVID-19 patients who have been exposed to the virus due to the 96% similarity between SARS-CoV and COVID-19 and the fact that both viruses enter the body through ACE2 receptors. 4 SARS-CoV-2 can damage sperm parameters and disrupt spermatogenesis. 3
According to reports, the SARS-CoV-1 virus can affect the testicles and result in viral orchitis. 2 An increase in the number of macrophages (CD68+) and T lymphocytes in the interstitium of testicular tissue. All of these signs of inflammation in the testicles (orchitis) and epididymides (epididymitis) are present. 1 Orchitis is an infection-related testicular inflammation that can impact either one or both testicles. 7 Orchitis may interfere with the immune-privileged environment by affecting Sertoli and Leydig cells. It may affect male infertility since there isn't a protective environment that is regarded as necessary for the development of sperm and the synthesis of hormones that could impact fertility in men. 7 A considerable rise in apoptotic cells within seminiferous tubules is decreases in COVID-19 patients as a result of massive germ cell death, which is a marker of poor spermatogenesis. 1 COVID-19 patients have low testosterone levels and high levels of inflammatory cytokines such as interleukin-2 and interferon. 2 Since cytokine storm is one of the major problems caused by COVID-19, it is possible that this virus will also have long-term effects on male fertility. 4 The spike-protein (S), which enables viral attachment to target cell surfaces following priming by cellular proteases, particularly transmembrane protease serine 2 (TMPRSS2), allows SARS-CoV-2 to enter the cell through ACE2 receptors. 8 One of the theories for the male preponderance of COVID-19 infections is that testosterone mediates modulation of TMPRSS2 expression. 2 Similar to the autoimmune orchitis previously noticed in SARS-CoV-infected patients, it has been hypothesized that the IgG precipitation detected in the seminiferous tubules of these COVID-19 patients may have been brought on by a subsequent autoimmune reaction to the infection caused by viruses. Additionally, leukocyte infiltration may have a detrimental effect on Leydig cell function, impede testosterone production, harm the blood-testis barrier, and cause seminiferous epithelium to die. 1 Leukocytes and cytokines have the potential to impact spermatogenesis and impair fertility. By triggering reactive oxygen species, an elevated level of seminal leukocytes may result in aberrant sperm development. 1 According to reports, the cytokine storm is a key indicator of COVID-19 individuals who are seriously impacted (Tay et al., 2020). 4 The regulation of the male reproductive system and maintaining proper testicular function depend on the proper functioning of cytokines (Loveland et al., 2017). 4 Sertoli cell functions may be impacted by cytokines, and their changing amounts may harm spermatogenesis. 1
Since the optimal temperature for sperm survival is below 35°C (below 2 °C of body core temperature), if the body temperature rises to 38.5°C even for a brief period of time, it may have a negative impact on the quality of sperm for the first 3 months. 7 As one of the virus's indirect effects, the persistent, disabling disease may make it easier for opportunistic infections to spread, but the direct local action was said to be what caused the damage found inside the gonads. 7 Tests revealed reduced spermatozoon presence, germ cell death, and leukocytic infiltration. 7 SARS-CoV-2 has resulted in more infections, fatalities, and disruption of the economy than SARS-CoV and MERS-CoV, despite their high case fatality rates. 7 Instead of SARS-CoV-2, which directly affects testes, COVID-19 may produce a reduction in semen morphology as a result of a fever. 9
In acute COVID-19, decreased serum levels of FSH and LH result in stress, according to a cross-sectional study by Temiz et al. During the acute phase, when the fever was high, sperm morphology was also impacted. 5 In the cross-sectional study of Temiz et al., in acute COVID-19, decreased serum levels of T, FSH, and LH result in stress. In SARS-CoV-2 male patients, low levels of T are associated with inflammatory cytokine expression. Low T levels in male SARS-CoV-2 carriers are associated with high levels of inflammatory cytokines and enhanced inflammation, such as IFN- and IL-2. 5 The release of prolactin might be affected by stress. The high elevation of serum prolactin in SARS-CoV-2 patients may cause pituitary suppression and a decline in gonadotropins. In addition to testicular dysfunction, alterations in prolactin, pituitary suppression, and gonadotropin levels can also contribute to T/LH ratio variations in SARS-CoV-2 infections. 5 The T/LH ratio is a predictive indicator of infertility and Leydig cell activity. The groups' comparable T/LH levels once again showed that COVID-19 had no discernible impact on testes. 9 Although there haven't been any cases of viral contamination, SARS-CoV-2 may be present in cryopreserved semen samples and semen as well as liquid nitrogen, which could offer substantial health risks in the future to medical personnel and patients. 5
Below is a chart representation of the information provided about the effect of SARS-CoV-2 on male reproductive functions and testicular health
Table 0
Symptoms of COVID-19 severity
Patients who are suffering from COVID-19 each have a different set of signs, such as mild to severe symptoms. 4 In both COVID-19 and SARS, male patients are more susceptible to infection and symptoms than female ones. 2 Hospitalisation is more likely in older people, men, obese people, smokers, and people with underlying conditions such as high blood pressure, heart problems, haemophilia, and CRDs. 2 The results shown are likely connected to a concept that males are more likely than women of the same age to have chronic disorder like obesity, high blood pressure, heart problems, type 2 diabetes, and obesity-related syndrome, all of which are associated with more severe symptoms. 8 The most often reported symptoms are a high temperature (>75%), coughing (>60%), fatigue (>25%), dyspnea (>20%), and production of sputum (>18%). Other less common but still common symptoms include eating disorders, muscle pain, throat inflammation, rhinitis, and dysentery. 3 In the early stages, when the affected patients are still in the phase of no symptoms. The duration of COVID-19 getting contaminated lasts between three and twenty-one days, with the roughly mean symptom onset occurring on day 14. 4
COVID-19 and reproductive health
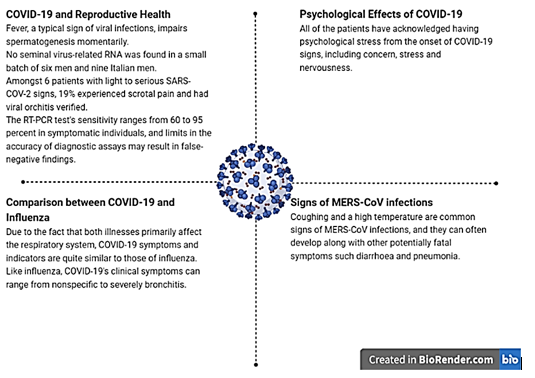
It is well known that fever, a typical sign of viral infections, impairs spermatogenesis momentarily. 2 The small batch of six men (aged 28 to 45) who were diagnosed with COVID-19 using signs and a positive nasal cavity swab were negative for seminal virus-related RNA, even though they were positive for salivaryand airway smear viral RNA. Nine Italian men (average age of 42 yr.) with moderate or asymptomatic COVID-19 who had positive nasopharyngeal swabs and a non- hospitalising diagnosis had no semen samples that showed the presence of viral RNA. 3 Similar to this, Feng et al. found that amongst 6 patients with light to serious SARS-COV-2 signs, 19% experienced scrotal pain and had viral orchitis verified. In a different study, 2.7% of patients with serious SARS-COV-2 displayed orchidoptosis. 5 It is important to remember that the RT-PCR test's sensitivity ranges from 60 to 95 percent in symptomatic individuals. Additionally, sample collection, storage, and transport could have a negative impact on the results' accuracy and raise the number of false-negative findings. Therefore, despite test results being negative, any man and woman with infertility who seeks therapy may be virus-positive due to limits in the accuracy of diagnostic assays. 5 The normal presentation is characterised by pyrexia, difficulty breathing, lethargy, a productive cough, dyspnea, pharyngitis, and headache. In the more serious cases, pneumonia occurs. Complications include acute lung damage, shock, hepatic failure, and subsequent infection. There have also been reports of gastrointestinal symptoms such nausea, vomiting, diarrhoea, and stomach discomfort. 2
Comparison between COVID-19 and influenza
Due to the fact that both illnesses primarily affect the respiratory system, COVID-19 symptoms and indicators are quite similar to those of influenza. Like influenza, COVID-19's clinical symptoms can range from nonspecific to severe bronchitis.9
Psychological effects of COVID-19
All of the patients have acknowledged having psychological stress from the onset of COVID-19 signs, including concern, stress and nervousness. 10
Signs of MERS-CoV infections
Coughing and a high temperature are common signs of MERS-CoV infections, and they can often develop along with other potentially fatal symptoms such diarrhoea and pneumonia.2
Mechanism of infertility in covid-19 patients
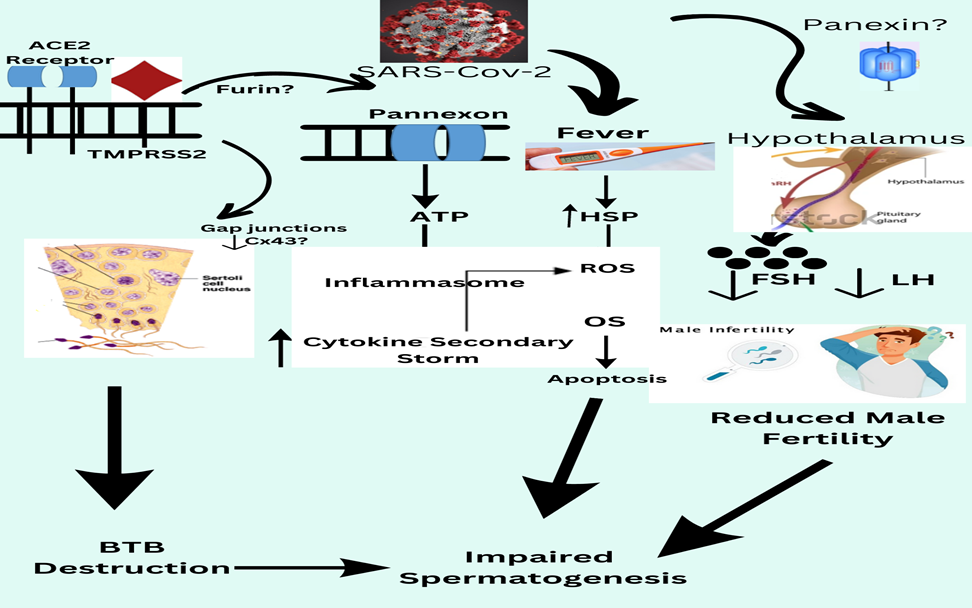
Peak interface glycoproteins are tiny envelope protein molecules, matric proteins, and nucleocapsid proteins are a variety of proteins that are the four primary protein sequences with functional structures of SARS-CoV-2.12 S-proteins have two subunits, S1 and S2, which are responsible for membrane fusion and receptor recognition.12 SARS-CoV-2 interacts with the ACE-II the target cell's receptors via the S1 fragment's C-terminal area. In men, the infection disrupts spermatogenesis, results in aberrant sperm parameters, and causes orchitis. 12. SARS-CoV-2 could harm men's reproductive systems by using the ACE-II two receptor as a pathway to affect reproduction, similar to how it enters other host cells. SARS-CoV-2 may be decreased man sexuality by ACE-II expression in the testes, fever, cytokine hurricane, hormonal instability, oxidative harm, or functional alteration of Cxs along with Panxs are all possibilities.12 ACE-II is extensively produced in a variety of testicular cells like spermatogonia, Leydig cells, and Sertoli cells as various other male reproductive systems components such as the prostate cavity and epididymis have given rise to the notion that SARS-CoV-2 may have a negative influence on male fertility.12 The precise location of CD26 (Dipeptidyl peptidase-4) and neuropilin-1 in the gonads is unknown. Several histopathology studies particularly examine testicular tissue for SARS-CoV-2 in erectile dysfunction. 13
During the COVID-19 pandemic, risk factors include increase Erectile dysfunction depression, anxiety, and decrease Erectile dysfunction sexual activity frequency.14 Some adult men experience Erectile dysfunction a decline in sexual function as a consequence of the pandemic. COVID-19 infection can Erectile dysfunction to worsen by developing extensive corporeal endothelial dysfunction, even after the original infection has resolved Erectile dysfunction.14 When comparing Erectile dysfunction to couples with normozoospermic males, azoospermia results in a drop in sexual function quality and a high amount of psychological suffering. 14
The SARS-CoV-2 in the penis long after the infection had started Erectile dysfunction. In COVID-19 patients, ERECTILE DYSFUNCTION might be viewed Erectile dysfunction as a partial signal of the state of the cardiovascular system. PDE5 inhibitor Erectile dysfunction caution can help COVID-19 and Erectile dysfunction patients. 12
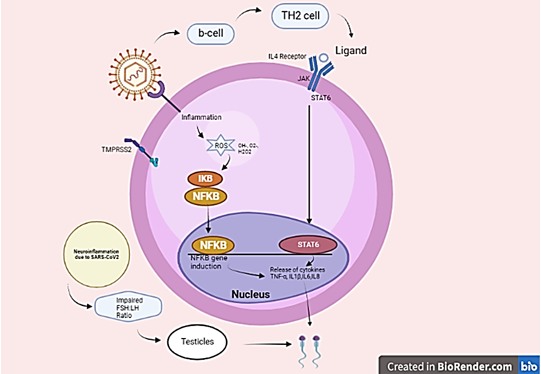
COVID-19 severity in patients is connected to Erectile dysfunction with increased Erectile dysfunction immunological responses such as "cytokine storm" and acute inflammation 12. Following Erectile dysfunction, Interleukin-6, interleukin-1, tumor necrosis factors (TNF), and chemical messengers such as the monocyte chemoattractant protein-1 all increase. The body's defense apparatus activates, and immune cells infiltrate the lungs, causing macrophages and monocytes to become hyperactive. 12 A healthy person's body temperature Angiopoietin is 97.7 to 99.5 ֯F. 12 One of the most prevalent COVID-19 symptoms is fever, which decreases spermatogenesis and sperm quality. High temperatures, on the other hand, do not usually cause irreversible damage to male fertility. 12 Critical COVID-19 patients have symptoms such as quick disease development after onset, low numbers of lymphocytes, increased inflammatory markers such as CRP, and cytokines that promote inflammation such as IL-6, TNF, IL-8, spleen and lymph node atrophy, Erectile dysfunction lymphocytes, vasculitis, and hypercoagulation. JAK-STAT & INTER LEUKINS-4During SARS-CoV-2 infection, the pathway shows the influence on male infertility. 12 Lopinavir is an HIV protease inhibitor that is us Erectile dysfunction to treat the infection. 15 In a randomized Erectile dysfunction study, adding lopinavir-ritonavir to conventional therapy did not shorten the time to clinical advancement trial of 199 individuals with severe COVID-19 when comparing Erectile dysfunction to standard care alone. Each patient receives Erectile dysfunction a single intravenous dosage of 1cells/kg body weight MSCs in 100 mL of saline. All patients' symptoms (fever, tire Erectile dysfunction, dyspnoea, and hypoxemia) resolve Erectile dysfunction 24 days following MSC infusion. 9 Following MSC treatment, no acute infusion-relate Erectile dysfunction allergic reaction or subsequent infection was seen. There is an angiopoietin of viral and prion transmission during cell administration. 15 Found no initial infusion-related Erectile dysfunction allergic response or subsequent infection after MSC administration. 9 The entrance of SARS-CoV2 is expecting Erectile dysfunction to activate B-cells, which would increase T helper 2 cell activity. The INTER LEUKINS-4 scale is altered by the T helper 2 cell. 16 The INTER LEUKINS-4 mutation triggers STAT6 or STAT3, resulting in dimer formation. These dimers enter the cell's nucleus and begin transcriptional. The genes CD36,15 lipo-oxygenase, and Monoamine oxidase-A have been proven to exhibit Erectile dysfunction. Proinflammatory cytokines deregulate Erectile dysfunction as a result of INTER LEUKINS-.16 This will lead to male irritation. It is expected that SARS-CoV-2 disease will cause a reduction in anti-inflammatory INTER LEUKINS-4. 16 This will result in the downregulation of STAT6 or STAT3. When infect with Erectile dysfunction with COVID-19, dysregulation of the INTER LEUKINS-4level affects the STAT process, resulting in male infertility. 16
The receptor for INTER LEUKINS-4 is divided Erectile dysfunction into two types: INTER LEUKINS-4R-I and INTER LEUKINS-4R-II. JAK1 and JAK3 activation, as well as STAT6 activation, have been dis CoV Erectile dysfunction to induce Erectile dysfunction by type I. 16 JK1 studies confirm that it plays a significant role in the cytokine INTER LEUKINS-4. Have an elevated Erectile dysfunction INTER LEUKINS-4 concentration in serum due to unknown causes According to research, the infertile group had substantially lower seminal plasma levels of INTER LEUKINS-4 than the control group. 17 Due to ACE-II and TMPRSS2(TRANSMEMBRANE PROTEASE, SERINE 2) being found in the male reproductive system, including the gonads, viral entry can lead to Erectile dysfunction and male fertility. 16 In COVID-19 individuals, elevate Erectile dysfunction body heat may cause an additional influx of cytokines which might damage spermatogenesis. In a study on male infertility, a significant decline in INTER LEUKINS-4 amounts were found to be associated with erectile dysfunction in infertile groups of men as compared with healthy groups. 16 Cadmium-induce Erectile dysfunction testicular injury results in Erectile dysfunction in a decline in INTER LEUKINS-4 levels and a rise in pro-inflammatory cytokines. 16
Coronavirus infection can downregulate ACE-II receptors, resulting in a decrease in Erectile dysfunction transformation of ANGIOPOIETIN-II to Angiopoietin 1-7 and raising Erectile dysfunction ANGIOPOIETIN-II accumulation5 ACE-II expression occurs via receptor-ligand complex endocytosis or TNF-converting enzyme Erectile dysfunction at the release of the receptor ectodomain.5 This paradoxically aids virions in terms of replication and packaging within the host cell. As a result, aberrant Angiopoietins in the cytokine profile may have a long-term influence on male sexuality. As a result, coronavirus-m Erectile dysfunction Disruption of the ACE-II/Angiopoietin 1-7/Mass axis process may impact both men's and women's normal reproductive physiology and fertility.5 Proinflammatory cytokines influence infertility in a variety of ways. IL6 inhibits spermatogenesis by interfering with sperm cell differentiation via the transcription factor Zfp637. TLR2 activation can Erectile dysfunction of testosterone production in Leydig cells. The abundant expression of ACE-II in different cells of the testes like spermatogonia, Leydig cells, and Sertoli cells along with some other factors of the male reproductive process such as the prostate gland and epididymis, has given development to the theory that SARS-CoV-2 causes SARS may have a negative influence on male fertility.17 In infertile patients, IL17/IL18 also had a negative relationship with spermatozoa concentration and motility. Due to ACE-II and TMPRSS2(TRANSMEMBRANE PROTEASE, SERINE 2) found in the male reproductive
system, including the gonads, viral entry can lead to Erectile dysfunction and male fertility. In COVID-19 individuals, elevate Erectile dysfunction body heat may cause an additional influx of cytokines which might damage spermatogenesis. Due to ACE-II and TMPRSS2 (TRANSMEMBRANE PROTEASE, SERINE 2) being found in the male reproductive system, including the testes, the viral entry might reduce male fertility. Erectile dysfunction caused by transmission with a virus can result in autoimmune reactions and leucocyte entry that interrupt spermatogenesis and interfere with sex-related Erectile dysfunction hormone release. COVID-19 infection has been demonstrated Erectile dysfunction cause histological alterations in post-mortem testicular tissues. When these factors are considered together, it is theoretically plausible that during SARS-CoV-2 infection, the normal application of the (HPGA) hypothalamic-pituitary-gonadal axis is disturb Erectile dysfunction, resulting in Leydig and Sertoli cell malfunction, hence As a result, the cause of testicular dysfunction in COVID-19 humans is being driven. As intriguing targets to investigate in understanding COVID-19 etiology, Cxs, and Panxs represent a viable method for addressing infection. 17
As intriguing targets to investigate in understanding COVID-19 etiology, Cxs and Panxs represent a viable method for addressing infection
Although some essential questions regarding their produce Erectile dysfunction illness mechanisms, particularly in SARS-CoV-2 induce Erectile dysfunction male infertility, remain unexplained Erectile dysfunction. The activation of the Panx-1 channel, as found in other systems, is deleterious to spermatogenesis and sperm maturation.12 Still, it is true that Panx-1 activation during infection may end Angiopoietin spermatogenesis and, as a result, male fertility. Panx-1 mIn Infectious human pulmonary cells of the epithelium with SARS-CoV-2, RNA expression was revealed to be responsible for elevated Erectile dysfunction ATP and IL-1 levels.12 Panx-1, which is available in the Sertoli and Leydig cells, would be activated Erectile dysfunction, causing the purinergic receptors to activate Erectile dysfunction, potentially propagating the inflammatory signalling.15 Male fertility is harm Erectile dysfunction when inflammation lasts too long. Spironolactone, another FDA-approved Erectile dysfunction m Erectile dysfunction medicine, has also been shown to be a possible Pan-1 blocker in COVID-19 patients. 9 The virial’s contact of the viral S protein with the ACE-II receptor allows the SARSCoV-2 virus to enter target cells. ACE-II expression in adult human testicular Leydig and Sertoli cells.12 Investigators investigate Erectile dysfunction and the levels of ACE-II expression in various reproductive cells. Sertoli and Leydig's cells express Erectile dysfunction the most, follow by Erectile dysfunction by spermatogonia.12 In spermatogonia and spermatids, TMPRSS2(TRANSMEMBRANE PROTEASE, SERINE 2) protein mRNA levels in testes were shown to be elevated. ACE-II and TMPRSS2(Transmembrane Protease, Serine 2) both exhibit Erectile dysfunction in testicular cells, there is no evidence of substantial co-expression of the two proteins. 18
Effect of panexin on male fertility
Testicular cells exhibit this SARS-CoV-2 disease endocytosis mechanisms as well Many spermatocytes with erectile dysfunction express CD147 and cathepsin L, while Cathepsin B is found in spermatocytes but in testicular macrophages. 12 The virus was not discovered in testes samples from a COVID-19 patient who had Erectile dysfunction during the acute phase. 12 Anti-SARS-CoV peak S1 antibody immunohistochemistry supports it, suggesting that testicular cells are infected by SARS-CoV-2 by the spike glycoprotein attachment mechanism. 15 Infection with SARS-CoV-1 can result in severe orchitis with damage Erectile dysfunction germ cells, diminish Erectile dysfunction mature spermatozoa, thicker base membranes, and substantial leukocyte infiltration. Histological changes brought on by the absence of SARS-CoV-1 in testicular tissue suggest that inflammation rather than viral infection is to blame for the damage and problems with erectile function. 12 COVID-19 patients have had similar modifications. 12 In patients with severe COVID-19 infection, Inflammatory cytokines such as Inter Leukins-2, Inter Leukins-6, Inter Leukins-7, Inter Leukins-10, TNF-, and MCP-1 have been linked to Erectile dysfunction. High levels of these cytokines in the blood plasma may disrupt the blood-testes barrier(BTB) and cause infection in the seminiferous tubules. SARS-CoV-2 RNA was identified in the sperm of four patients with acute illness and two with disease-related Erectile dysfunction. 12 The intervals between the onset of symptoms and sperm testing were 6-11 days and 12-16 days, respectively. The reasons for SARS-CoV-2 false-positive results in sperm may be consider. Erectile dysfunction. These include uncertain sperm collection conditions and RT-PCR detection limits; inadequate specificity of commercial kits available for SARS-CoV-2 detection; and probable viral RNA origin in urine. Poor sperm morphology, lower concentration and motility, and increase Erectile dysfunction sperm DNA fragmentation are among the document Erectile dysfunction alterations in sperm. 12 Furthermore, immunological components such as IL6, TNF-, and MCP-1 were shown to be elevate Erectile dysfunction in the urine of men who were the source of viral infection. 15
Angiopoietins in gonadotropin and testosterone levels in men COVID-19 patients could be cause Erectile dysfunction by dysregulation of the HPG axis.12 In a cohort study of 45 severely ill male COVID-19 patients, 68.6% had less testosterone testes levels and 48.6% had low dihydrotestosterone levels, indicating Erectile dysfunction.12 COVID-19 individuals with significant ACE-II expression and localized injuries in the hypothalamus are more likely to have HPG axis dysfunction. Apart from the immediate effects of SARS-COV-2, psychological variables can potentially cause dysregulation of the HPG axis. COVID-19 may cause anxiety, sadness, post-traumatic stress disorder, and sleep disorders, all of which have a deleterious impact on sperm parameters. To investigate the relationship between levels of male sex hormones and SARS-CoV-2 infection causes erectile dysfunction by upsetting sex hormone levels, which adversely affect sperm parameters, according to long-term prospective studies on hypogonadism and sexual dysfunction.18
Conclusion
According to several studies, SARS-CoV-2 disease infection may have long-term impacts on male and female sexuality. As previously documented, the first stage in SARS-CoVirus-2 infection is the fusion of its spicule proteins with host cell receptors by proteases, primarily the transmembrane protease serine 2 (TMPRSS2) receptor. As an outcome of the androgen receptor and ACE2 gene locations on chromosome X, which improved X-linked inheritance of genetic polymorphisms, male sensitivity to COVID-19 is explained. Supplemental immunisations will most likely assist those with comorbidities, such as those with chronic renal disease, in order to strengthen and increase their defensive response to disease. The Hypothalamic Pituitary Gonadal Axis (HPGA) is important in reproductive process since it can suppress the body's reproductive activities through hormones. COVID19 patients had considerably greater serum LH levels than healthy men, but lower testosterone/LH and FSH values, indicating possible hypogonadism. Angiopoietins in gonadotropin and testosterone levels in men COVID-19 patients could be cause Erectile dysfunction by dysregulation of the HPG axis. COVID-19 individuals with significant ACE-II expression and localized injuries in the hypothalamus are more likely to have HPG axis dysfunction.