Introduction
Osteoporosis (OP), a metabolic bone disease, reduces bone density and microstructure, hence increasing the vulnerability of bones to fractures. A worldwide epidemiological study on spinal osteoporotic fractures indicates that half of males and one-third of women over the age of 50 will eventually suffer with osteoporosis.1, 2 Studies on the matter estimate that osteoporotic hip fractures in Asia would cost more than $15 billion annually by 2025.3 Reducing the load on worldwide healthcare systems and avoiding fractures depend on early identification and treatment of osteoporosis.
These cells remove damaged bone and simultaneously produce new bone in a sequential manner, therefore assisting in bone healing. Bone marrow mesenchymal stem cells (BMSCs) become osteoblasts; monocyte-macrophage fusion generates osteoclasts.4, 5, 6
A range of secondary ailments, including autoimmune diseases, rheumatism, gastrointestinal difficulties, blood disorders, neurological conditions, ageing, and endocrine problems, can also induce OP. Figure 1 Additional major risk factors for osteoporosis are lifestyle choices including inadequate vitamin D and calcium intake, too much salt consumption, too much alcohol usage, and smoking.7 These components could upset the fine equilibrium between bone cell activity and the dynamic cycle of bone formation and absorption. This can aggravate both osteostasis and OP.8
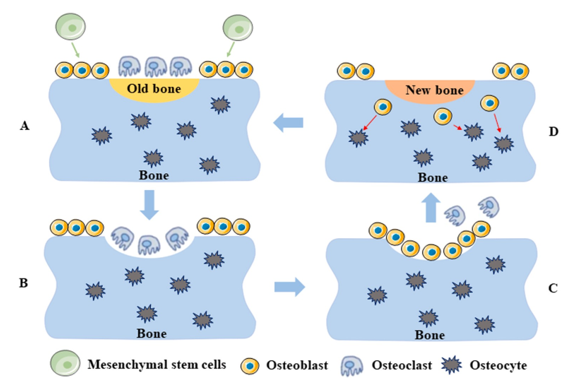
A. The final end-products of mesenchymal stem cell differentiation are osteoblasts and osteoclasts. B. As they are the ones in charge of resorbing bone, osteoclasts migrate to the surface of deteriorating bone. C. Osteoblasts rush to the surface to initiate bone creation the second the osteoclasts depart. a. In order to maintain the bone's quality and strength, new bone is used to replace old bone.
In terms of the drugs used to treat or prevent osteoporosis, there are essentially two main classifications. The first group includes medications that prevent bone resorption, whereas the second group includes conditions that encourage bone formation.9 Research has shown that several osteoporosis drugs effectively restore bone strength; nevertheless, some treatments also lower bone strain unintentionally.10 In addition, there are a number of expensive and time-consuming clinical drugs that come with serious side effects and necessitate long-term use.11 Take bisphosphonates as an example; they can cause bone fractures if used in excess, and hormone therapy can have serious side effects including thrombosis.12 Traditional Asian medicine has made extensive use of the miraculous ginseng plant for thousands of years for a wide variety of medical issues. It is a popular medical herb with many different pharmacological components; it is also a food related herb. Among these ingredients are bioactive proteins, peptides, polyacetylene, oligosaccharides, and saponins. Ginsenosides can be grouped into two types: protopanaxadiol (PPD) and proto-panaxatriol (PPT). 13 Although ginsenoside is one of the active components of ginseng, it is just one of many chemicals extracted from the root of the plant. 14 An example of a ginsenoside is Rb1, followed by Rb2, and so on along the chain to Rb3, Rc, Rd, Rh2, Rg3, and finally F2. However, there are ginsenosides of the PPT type, such as Re, Rf, Rg1, Rg2, and Rh1, that are believed to provide therapeutic promise for a range of diseases. 15, 16 The number of ginsenosides may have therapeutic benefits on osteoporosis by controlling the activity of osteoclasts and osteoblasts, according to an increasing amount of cell tests, animal experiments, and clinical investigations conducted in recent years. 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30 The results of recent scientific studies corroborate these conclusions. Thus, from the perspectives of signal pathways, bone mesenchymal cells, osteoblasts, and osteoclasts, this investigation will delve more into the mechanism of action of ginseoside in the treatment of osteoporosis. The goal of this review is to outline the objectives of new ways of treating osteoporosis.
Different types of ginsenoside compounds
nd Figure 2 respectively present the active components that are connected with the ginsenosides that are utilised in the treatment of OP.
Figure 2
Different ginsenosides affect different parts of the anti-osteoporosis mechanism's signalling pathway.
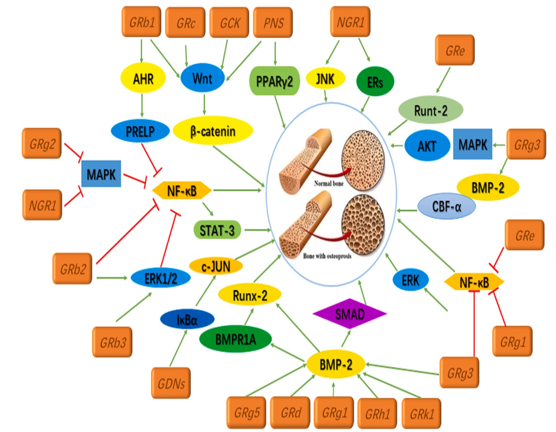
Table 1
Ginsenoside properties in the treatment of osteoporosis.
Bone marrow stromal cells (BMSCs
Mesenchymal stem cells (MSCs) found in bone marrow are known as bone marrow stem cells (BMSCs). 59 The capacity of these MSCs to develop into osteoblasts, adipocytes, or chondrocytes is crucial for their function in preserving normal bone stability. It has been demonstrated that osteoporosis develops in part because of changes in bone marrow stem cell (BMSC) proliferation and differentiation, quantity, and function. 60 (Figure 3).
Figure 3
Certain ginsenosides activate BMSCs in a way that promotes bone development and inhibits cell death.
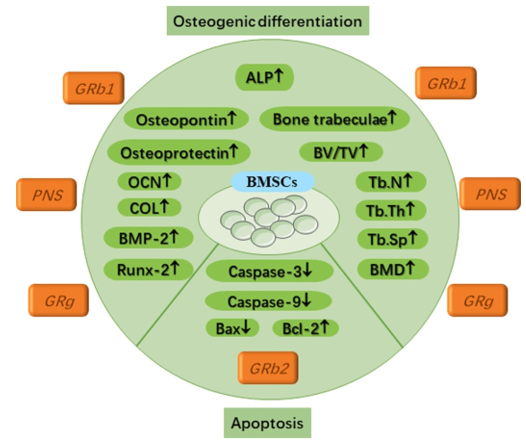
Supporting the process of bone marrow stromal cell osteogenic differentiation
The outcome of this transformation is a decrease in bone production, which happens when the differentiation of osteoblasts from bone marrow stem cells (BMSCs) is relatively low. This crucial biological function has thus been the subject of several experimental investigations, some of which are described below. Calcium deposition and alkaline phosphatase activity (ALP) in BMSCs cultured with Rb1 for seven days increased in a concentration-and dose-dependent manner. 61 This happened when the dosage and concentration of Rb1 were raised. Furthermore, their study's results showed that Rb1 induced osteoblast differentiation by significantly raising the expression levels of osteogenesis-related proteins such osteopontin and osteoprotegerin. It is appropriate to address the function of ginsenoside Rg1 in bone tissue restoration in light of a recent study that examined the regulation of periodontal tissue using hydrogels loaded with Rg1. 62 Cellular research shown that Rg1 inhibited cell death (apoptosis) and significantly facilitated the growth of bone marrow stem cells (BMSCs). 63 Increased expression of genes linked to osteogenic differentiation is a consequence of Rg1's role in the BMP-2/Smad signalling pathway. The following genes are part of this set: ALP, COL1, BMP-2, and Runt-related transcription factor-2 (Runx2). The results of the animal studies showed that rats given Rg1 had much higher levels of bone trabecular ratio, trabecular number, trabecular thickness, bone mineral density, and bone volume % compared to rats given a placebo. The number of bone trabecular separations (Tb. SP) was, however, markedly reduced.
Evolution has spared several signalling pathways, and the Wnt signalling system is one such them. Cell proliferation, differentiation, and destiny are all controlled by this pathway. Illnesses including cancer, osteoporosis, and congenital disability can arise when Wnt signalling is manipulated in an improper way. To learn how ginsenoside works to treat osteoporosis, researchers have looked into the Wnt signalling pathway. 64 By promoting the proliferation and osteogenic differentiation of bone marrow stem cells (BMSCs), the peripheral nervous system (PNS) contributes to the stimulation of bone production. Osteoprotegerin (OPG), β-MRNA synthesis of catenin, and cyclin D1 are all improved, however RANKL and PPARγ2 mRNA expression are decreased. 65 The Wnt/β-catenin signalling pathway, in contrast, reduces the expression of RANKL/OPG. 66 This decrease in expression aids in protecting the skeletal system by limiting bone resorption. Furthermore, studies have shown that ginsenoside compound K (CK) can stimulate osteogenic differentiation in bone marrow stem cells (BMSCs) via activating the Wnt/β-catenin signalling pathway. 67
Inhibiting the apoptosis of bone marrow stromal cells
Figure 1 shows a schematic of the physiologically-driven bone remodelling process. Differentiation begins with mesenchymal stem cells and culminates in osteoblasts and osteoclasts. B. Bone resorption occurs when osteoclasts, the cells responsible for the process, move to the surface of dead bone. C. The initial phase in making new bone, known as osteoblast migration to the surface, occurs immediately after osteoclasts depart. a. Removing and replacing old bone with new bone is necessary to keep the bone's quality and strength.
Bone marrow stem cells (BMSCs) are potential therapeutic targets for osteoporosis due to age-related changes in cell death and differentiation. Bone marrow stem cell (BMSC) transplantation, apoptosis inhibition, BMSC modification of differentiation capacity, and BMSC elimination are all successful treatments for osteoporosis. Future research can build on these discoveries in innovative ways. 68
Osteoblast
There are four stages that osteoblasts go through as they produce bone: proliferation, maturation of the extracellular matrix, mineralisation of the extracellular matrix, and apoptosis.69 The process culminates in apoptosis. As the number of osteoblasts increases during proliferation, new bone matrix is formed, type I collagen is synthesised and secreted, and many layers of cells are formed(Figure 4). 70
Figure 4
Different ginsenosides play specific roles in osteogenic differentiation, anti-oXidative stress, anti-inflammation and anti-autophagy through osteoblasts.
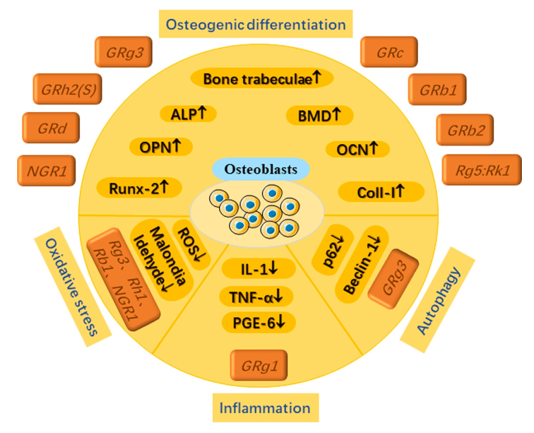
Promoting the activity of osteoblasts
An increase in the number of calcium nodules and consequent mineralisation can be achieved by Rb1 by a dramatic overexpression of Runx2, osteopontin (OPN), and osteocalcin (OCN) proteins in osteoblasts. In addition, the regulatory approach mentioned earlier is carried out by using three genes from the database to activate the AHR/PRELP pathway and inhibit the nuclear factor kappa-B (NF-κB) route. 71 Furthermore, several studies have shown that the Wnt/β-catenin signalling pathway can be used to acquire three microorganisms produced from Rb1 fermentation. Among these microbes, you can find two novel dehydrogenation metabolites and a well-known 229-ketone derivative. These microbes improve osteoblast differentiation indicators in a way that depends on the dosage.72 Beyond Rb1, further members of the Rb family, such as Rb2, have demonstrated strong anti-osteoporosis properties. Several studies have shown that Rb2 therapy significantly reduced bone loss in cancellous bone while retaining biomechanics. Consequently, rat bone volume fraction went raised from 2.2% to 6.0%. While Rb2 increased serum levels of bone alkaline phosphatase (BALP), decreased levels of tartrate-resistant acid phosphatase (TRACP), increased levels of octanoin (OCN), and decreased levels of tyrosine phosphatase (TRAP) and PPAR-γ, these changes occurred simultaneously. Also, Rb2 did a better job than cathepsin K of preserving cancellous bone's biomechanical strength, postponing bone loss, blocking bone resorption, and inducing osteogenic differentiation. These findings provide credence to the idea that ginsenosides can be used as an alternate treatment for osteoporosis. 73
There was a dose-dependent increase in the activity of proteins involved in osteoblast development and differentiation by the Rg5: Rk1 combination. Calcium deposition, extracellular mRNA expression of bone morphogenetic protein-2, levels of Runx2, and ALP activity were among the proteins that comprised this set of osteoblast indicators. 74 Rg3 significantly boosted MC3T3-E1 cell bone formation and differentiation. There was a lack of specificity regarding the signalling route that was involved, though. 75 Rg3 effectively counteracted dexamethasone's effects on bone mineral density and weight loss via the BMP-2/BMPR1A/Runx2 signalling pathway. Furthermore, Rg3 lowered markers of bone resorption and raised those of bone growth. Similarly, Rg1 can increase osteoblast numbers, ALP activity, and intercellular cAMP concentration, and it can compensate for the loss of BMD in the lumbar spine and tibia of rats that have undergone ovariectomy. 75, 76
Furthermore, other researchers have used components from different ginsenoside races with lower composition for their studies, in addition to the common ginsenosides already mentioned. According to research published in 2011 by Kim et al., Rh2 (S) can activate the PKD/AMPK signalling pathway, which in turn stimulates mineralisation and differentiation in MC3T3-E1 cells. While limiting osteoclast differentiation, ginsenoside Re promotes osteoblast differentiation, a process regulated by the crucial signalling protein Runt-2.Fundamental studies were also conducted on Rd in 2011 by the study team. 77 Through stimulating the AMPK/BMP-3/Smad signalling cascade, our experiments showed that Rd may promote mineralisation and differentiation of MC3T1-E2 cells. According to another study, Rc can greatly prevent bone density loss, bone volume percentage, and trabecular meshwork from decreasing and trabecular meshwork separation from increasing. In addition, it can regulate the catenin signalling system through the Wnt/β pathway, which implies it can enhance the expression of genes associated with bone production. 78
Panax notoginseng, a plant used in traditional Chinese medicine, is the source of the natural triterpenoid saponin molecule known as notoginsenoside R1, or NGR1. It may stimulate the formation of osteoblasts with great efficacy. Two key protein targets have been identified so far for this saponin's investigation. The capacity of NGR1 to induce concentration-dependent overexpression of miR-23a regulates Runx2 and OsX expression, ALP activity, and other genes in a positive way. In 2019, Wang et al. found one of these microRNAs, miR-23a. This technique is accomplished by utilising the mitogen-activated protein kinase (MAPK) and JAK23/STAT1 pathways, which are known to promote osteoblast growth successfully. The oestrogen receptors (ERs), which have been found to have oestrogenic properties, are another significant target. 79 "Oestrogen receptors" are another key area of focus. NGR1 activates the transcriptional activity of phosphorylated oestrogen response element (pERE)-luciferase-α phosphorylation, which in turn induces endoplasmic reticulum (ER) in osteoblasts. The transcription of osteoblast genes (such as COL1, osteonectin, osteocalcin (OC), Runx2, and osteriX) and alkaline phosphatase activity are both enhanced by this action, which is a biomarker for osteoblast development. Plus, it promotes osteoblast mineralisation. 80
Most ginsenosides still fight osteoporosis by getting osteoblasts to work more by hitting many targets and signalling pathways crucial for protein expression. I believe that bioinformatics might be chosen to explore the large gene database in order to carry out more effective and broad research.
Preventing Osteoblast Oxidative Stress
In order to increase the levels of mitochondrial respiration and ATP generation, osteoblast precursor cells often undergo metabolic changes. This is because osteoblast growth is energy intensive. In order to make sure there's enough power, this is done. However, when ROS levels are high, osteoblast differentiation is reduced, antioxidative ability is decreased, and ROS levels are increased. This vicious cycle continues until the osteoblast differentiation potential is lost or reduced.81 A large number of researchers have conducted experiments to prove this crucial mechanism. Rg3 can reduce malondialdehyde and reactive oxygen species (ROS) levels while increasing glutathione peroxidase and superoxide dismutase activity, which inhibits oxidative stress in rat bones.82 In contrast, Rg3 can promote bone formation and prevent bone resorption in addition to significantly up- or down-regulating associated proteins. Sera COL-I, osteocalcin, osteopontin, and bone alkaline phosphatase activity make up the first group. The second group includes tartrate-resistant acid phosphatase activity in serum and the N-terminal and C-terminal cross-linked peptide content of COL-I. As a result, Rg3 likely has the potential to alleviate AlCl3-induced osteoporosis. Research by Zhu et al. (2016) using the same animal model yielded similar results.83 Nevertheless, by utilising Rb1, they learnt that Rb1 can greatly enhance osteoblast ultrastructural features and the expression of osteoblast growth regulating factor mRNA. Additionally, oxidative stress generated by AlCl3 was discovered to be inhibited by Rb1. Also, studies have shown that Rh1, Rb2, and NGR1 can reduce oxidative stress in osteoblasts, which in turn increases the production of associated osteogenic markers. The ALP, Col-I, and OCN markers are among them. These medications show great promise in treating osteoporosis (OP).84, 85, 86
Reducing osteoblast inflammation
The balance of bone mass is ensured by osteoblasts, which are cells that are vital for the formation of new bone. Simultaneously inhibiting osteogenesis and activating inflammatory responses are possible outcomes of inflammatory stimulation. The inhibitory effects of osteogenesis and mineralisation are significantly affected by the high levels of inflammation in osteoblasts. 87 The levels of prostaglandin (PGE6) and TNF-α in osteoblasts can be significantly decreased by using various amounts of Rg1.88
Titanium particles can cause osteoblasts to experience a dramatic rise in inflammatory levels. Similar levels of IL-1 expression were seen in cells cultured just with titanium particles and in control cells. Inflammation levels in osteoblasts have been the subject of very little research up to this point. One of the areas where future research will concentrate on this as well. Investigating the potential of limiting the inflammatory expression of osteoblasts at a deeper level can improve osteogenic differentiation and boost the production of proteins relevant to osteogenesis.
Preventing autophagy in osteoblasts
The process of cellular autophagy is crucial to many biological processes, including the development and maintenance of living things. A process called "autophagy" involves the breakdown of various components within the cell, including organelles, proteins, and cytoplasm, into smaller structures called "autophagic endosomes" that are housed within endosomes. Finally, autophagic lysosomes are formed when autophagic endosomes combine with lysosomes. To achieve cellular homeostasis and organelle renewal, these lysosomes must degrade the materials they carry. 89 All the experts agree that a better way to stabilise bone metabolism is to activate autophagy levels in osteoblasts. The current state of osteoblast differentiation and mineralisation is greatly influenced by their autophagy level, which is why this is the case. The effects of ovariectomy (OVX) on body weight gain, bone mineral density decrease, and femoral tissue histological abnormalities were significantly mitigated by Rg3. Osteogenesis and autophagy were also significantly enhanced by Rg3. Rg3 may mitigate OVX-induced osteoporosis via the AMPK/mTOR signalling pathway.
Osteoclast
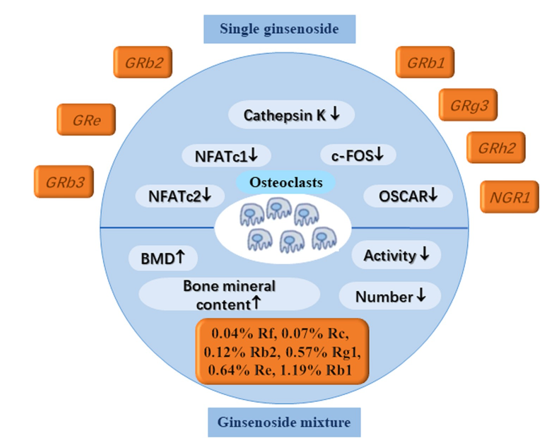
Large, multinucleated cells called osteoclasts are made in the bone marrow by merging mononuclear macrophages with myeloid progenitor cells. Precursors of osteoclasts are proliferative early immature mononuclear phagocytes. These early-stage osteoclasts enter the bloodstream in response to chemical cues. Finally, they enter the bone structure cavity, where basal multicellular units emit signalling substances that affect them. A wide range of pharmacological substances, transcription factors, cytokines, and other signalling factors can activate osteoclasts by causing their progenitors to fuse into multinucleated cells. The process of bone resorption is carried out by a particular type of cell in bone tissue called bone resorbing cells, often called osteoclasts. There is no difference in function between osteoclasts and osteoblasts. When it comes to bone growth and formation, the interplay between the two is crucial. 90 The bone microenvironment contains osteoclasts, which are macrophages with many nuclei. The components that make them up are M-CSF and NF-κB ligand receptor activator (RANKL), both of which are recognised as drivers of monocyte growth. 91, 92 As a result, numerous researchers in the US and abroad have studied the efficacy of ginsenosides as an osteoporosis treatment by manipulating osteoclasts (Figure 5).
Single ginsenoside
Most studies done thus far have focused on specific ginseosides, such as Rb1, Rb2, Rg3, Rh2, and Re. Rb1 can block the activities of NF-κB, p38 MAPK, and RANKL-induced c-Jun N-terminal kinase (JNK). 93 (In order to combat osteoporosis, it is necessary to activate a pathway that stops the formation of Raw264.7 osteoclasts by limiting the gene expression of c-Fos and nuclear factor of activated T cells (NFATc1) in these cells. The RANKL-induced MAPK pathway is blocked by Re, Rb3, and NGR1, which in turn limits osteoclast differentiation. According to previous research, Rb2 has a dose-dependent effect on the expression of NFATc2, c-Fos, and cathepsin K, which are genes that are known to be osteoclast marker genes. The suppression of the STAT3 signalling pathways and RANKL-induced NF-κB activation is linked to this mode of action, though. By downregulating the p38, extracellular signal-regulated kinase, and JNK pathways, Siddiqi et al. (2015) found that Rg3 reduces RANKL-induced osteoclast-specific markers. On top of the signalling channels already covered, here is another one. 94, 95, 96 The modulation of ERK phosphorylation is one of the essential mechanisms via which ginsenoside exerts its anti-osteoporosis effect. Not only that, but this method supplements the previously mentioned critical protein factors. 97 The non-cytotoxic Rh2 (S-type) can inhibit osteoclasts by suppressing RANKL-mediated MAPK and ERK activation. 98 It was also believed that Rh2 (R-type) inhibited osteoclasts.
Ginsenoside mixture
The osteogenesis of ginsenoside combinations is an area that has seen very little investigation thus far. Rb1 and Rg1 are more effective together than individually in blocking osteoclast differentiation, and the GDN has a high concentration of both. In a mouse model of lipopolysaccharide-induced bone resorption, GDNs were expressed by means of RANKL/IκBα expression. The c-JUN signalling pathway has a long-lasting inhibitory effect on osteoclast formation. 99 A combination of siX ginseng soaps containing 0.04% Rf, 0.07% Rc, 0.12% Rb2, 0.57% Rg1, 0.64% Re, and 1.19% Rb1 successfully inhibited osteoclast activity. First, this combination can reduce the number of osteoclasts. Additionally, it can enhance the production of mRNAs for the calcium receptor (Cal-R) and the oestrogen receptor-α (ER-α). This, in turn, influences the biochemical characteristics and structure of bones, leading to regulation of bone density and mineral content.100
Prospects of ginsenosides in the treatment of osteoporosis
The osteogenesis of ginsenoside combinations is an area that has seen very little investigation thus far. Rb1 and Rg1 are more effective in inhibiting osteoclast differentiation when present in GDN in substantial fractions compared to when used alone. In a mouse model of lipopolysaccharide-induced bone resorption, GDNs were expressed by means of RANKL/IκBα expression. The c-JUN signalling pathway has a long-lasting inhibitory effect on osteoclast formation.99 A combination of siX ginseng soaps containing 0.04% Rf, 0.07% Rc, 0.12% Rb2, 0.57% Rg1, 0.64% Re, and 1.19% Rb1 successfully inhibited osteoclast activity. Their analysis confirmed this. First, this combination can reduce the number of osteoclasts. Additionally, it can enhance the production of mRNAs for the calcium receptor (Cal-R) and the oestrogen receptor-α (ER-α). This, in turn, influences the biochemical characteristics and structure of bones, leading to regulation of bone density and mineral content. 100
Conclusion
There has been a rise in the amount of research conducted in recent years regarding the use of ginsenosides as a therapy for osteoporosis. A summary of the distinct signalling pathways or key factor mechanisms that are associated with bone marrow stem cells (BMSCs), osteoblasts, and osteoclasts is provided in this review. As a result, this review covers the possible mechanisms of various types of treatment for osteoporosis from three different perspectives. However, this treatment method is still in the preliminary stages of clinical transformation, and there are still a great deal of obstacles that stand between its experimental results and its clinical application. These obstacles include combination therapy, drug delivery pathways, bioavailability, and the combination of biochemical materials. Ginsenosides have the potential to become a new medicine for the treatment of osteoporosis and the promotion of fracture healing. Additionally, they serve as a powerful candidate for cytokines in tissue engineering bone. This is due to the growing attention that is being paid to the role that ginsenosides play in bone rebuilding.