Introduction
The oral route of drug administration is the most advantageous route due to ease of administration, non-invasiveness, adaptability, patient compliance and acceptability. Regarding the oral route of drug delivery, many substitutes have been continuously presented using the latest new technologies for pediatric, geriatric, nausea and non-adherent patients. Orally disintegrating films (ODFS), when placed on the tongue, immediately hydrate by soaking saliva upon disintegration and/or dissolution releasing the active pharmaceutical agent from the dosage form. ODFS are types of preparations that are commonly prepared using hydrophilic polymers allowing rapid dissolution upon contact with saliva.1
Research and development in the oral drug delivery segment has led to a shift in dosage forms from simple conventional tablets/capsules to modified release tablets/capsules to the oral disintegrating tablet (ODT) to the recent development of the oral strip (OS). In principle, an OS can be thought of as an ultra-thin strip about the size of a postage stamp with an active substance or active pharmaceutical ingredient and other excipients. The advantages of portability of the dosage of OS have led to a wider acceptability of this dosage form in both the pediatric and geriatric populations. 2
It has been known for centuries that solutes are rapidly absorbed when administered orally (buccal and sublingual administration). This absorption occurs via the reticular vein, which lies beneath the oral mucosa and is transported via the facial veins, the internal jugular vein, and the brachiocephalic vein. The absorbed drugs are then discharged into the systemic circulation. Therefore, oral administration of drugs can be used to bypass first-pass metabolism of drugs in the liver. The oral cavity regions offer higher absorption of drugs in the systemic circulation.
Formulation of a fast-dissolving film involves the application of both aesthetic and functional properties such as strip-forming polymers, emollients, active pharmaceutical ingredient, sweeteners, saliva stimulating agent, flavorings, dyes, stabilizers and thickeners. From a regulatory perspective, all excipients used in the formulation of oral medication strips should be approved for use in oral pharmaceutical dosage forms. 3
Materials and Methods
Materials: Teneligliptin hydrobromide hydrate was obtained from smruthi organics limited A-27, MIDC-Chincholi, Solapur.
Table 1
Method
General procedure for preparation of oral disintegrating film 4, 5, 6, 7, 8
The Oral disintegrating film of teneligliptin using polymers were prepared by solvent casting method. An aqueous solution of polymers was prepared in distilled water by soaking for 1 hr. & it was stirred with magnetic stirrer until polymer was completely dissolved. Teneligliptin was added to the aqueous polymeric solution and mixed thoroughly with magnetic stirrer. This was followed by addition of plasticizers like propylene glycol.
Thereafter, Sweetener like sacchrine was also added to the above solution. Citric acid was also mixed with it. The solution was mixed for 1 hour using magnetic stirrer so as to get homogeneous solution. After the final solution was placed on ultra-sonication for 1hr (to remove the air bubble). The solution was casted on a petri dish (diameter 9 cm) and dried at room temperature for 24 hr. The film was carefully removed from Petri dish, checked for any imperfections and cut into the required size to deliver the equivalent dose (2x2cm) per strip and packed in self-sealing covers and stored in a desiccator until further analysis.
Characterization of Oral disintegrating film
Physical appearance 5
The prepared oral films were evaluated for color, peel-ability, transparency, homogeneity, and flexibility.
Thickness of film 9, 10
Thickness of film was measured using digital Thickness gauge with a least count of 0.01 mm. The thickness was measured at different strategic locations. Each film was measured at 5 positions (center and four corners) and the mean thickness was calculated. This is essential to determine uniformity in the thickness of the film as this is directly related to accuracy of dose in the film.
Tack/Dryness test 11
Tackiness was determined by gently pressing the film between fingertips and noting whether it was tacky or non-tacky.
Weight variation test 12
For weight variation three films of every formulation were taken individually on digital balance then average was calculated.
Folding endurance 9, 10
Folding endurance was determined by repeatedly folding one film at the same place until it broke. The number of times the film folded at the same place without breaking or cracking gives the value of folding endurance.
Moisture content 13, 14
The prepared films were weighed and kept in a desiccator containing anhydrous calcium chloride. The films were weighed repeatedly until they showed constant weight. Percent moisture content was determined using the formula,
pH value 15
The pH value was determined by dissolving the film in 10 ml of distilled water. The pH value was recorded after bringing the electrode of the pH meter into contact with the surface of the formulation and allowing equilibration for 1 minute. All determinations were performed in triplicate. The film must have an almost uniform pH value.
Drug content16, 17
Drug content for all batches was determined by dissolving a 2 x 2 cm2 strip in 50 mL of dissolution medium. The solution was filtered through Whatman filter paper and analysed with a UV-spectrophotometer at 241 nm. Content uniformity tests were performed in triplicate for each batch of film.
Disintegration time,18, 19, 20
The in vitro disintegration time was determined visually in a glass beaker with 25 mL of distilled water with stirring every 10 seconds. Breakdown time is the time when the film begins to break or disintegrate. There are no established guidelines for estimating the disintegration time of orally rapidly disintegrating films.
In-vitro drug release
For in-vitro dissolution studies, drug release studies were determined by Franz Diffusion Cell Apparatus having external diameter is 3 cm, internal diameter is 2.8cm, the height of diffusion cell apparatus is 8cm and volume is 30ml. The receptor compartment maintained at 37ºC was continuously stirrer at 50rpm. Sample of 1ml was withdrawn at a predetermine time interval and replace with an equal volume of the dissolution medium equilibrated at the same temperature. The drug concentration of the withdrawn sample was determined by UV Spectrophotometer at 241 nm All studies were carried out in triplicate for each batch of the film sink conditions were maintained throughout the study.
Results and Discussion
Table 2
Study of organoleptic properties
|
Sr. no |
Test |
Specification |
Observation |
|
1 |
Appearance |
A white to off white powder |
Complies |
|
2 |
Taste |
Slightly Bitter |
Complies |
|
3 |
Odour |
odourless |
Complies |
Table 3
Melting point determination
|
Sr. no |
Melting point of teneligliptin hydrobromide |
|
|
|
Standard value |
Observed value |
|
1 |
218 oC |
208 oC |
The melting point was carried out by using capillary tube method. Melting point of teneligliptin was found to be 208oC.
Identification of Drug by λmax
Determination of Maximum wavelength of teneliglipitin
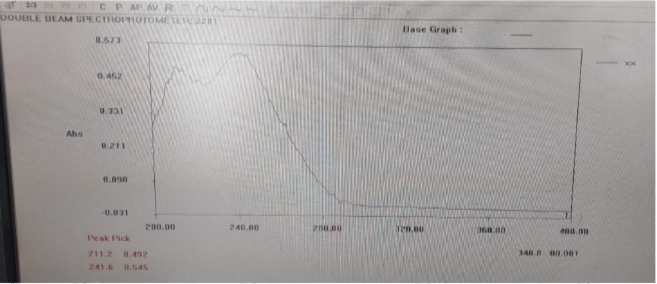
The wavelength of maximum absorption of the drug was determined by preparing stock solution of drug in buffer solution pH, scanned in the range of 200-400 nm and absorbance was measured by UV-Spectrophotometer (Systronic, UV 2201) and it was found that drug showing maximum absorption at 241.6nm
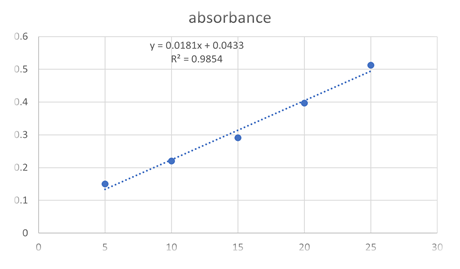
Calibration curve of teneligliptin in water
Teneligliptin showed maximum absorption at wavelength 241.6 nm in water. Standard curve was plotted by taking absorption of diluted stock solution (5,10,15,20,25 µg/ml) at wavelength of 241.6nm.
Physical appearance
In the following table, Visual appearance, color, Tack test were checked.
Table 5
Physical appearance of teneligliptin strip
Evaluation parameters for physical appearance of films
Physical parameters were checked simply with visual inspection of oral disintegrating film and by feel or touch.
Table 6
Thickness for different formulations of teneligliptin
|
Formulation code |
F1 |
F2 |
F3 |
F4 |
F5 |
F6 |
|
Thickness (mm) |
0.002 |
0.004 |
0.003 |
0.004 |
0.004 |
0.005 |
Thickness of fast dissolving film depends on the concentration of polymer. The thickness of the oral disintegrating films were measured using digital thickness gauge and the average thickness of all films is given in above table.
Weight variation
Table 7
Weightvariation of different formulations of teneligliptin
|
Formulation code |
F1 |
F2 |
F3 |
F4 |
F5 |
F6 |
|
Weight Variation |
43 |
36 |
43 |
45 |
39 |
47 |
The weight of Fast dissolving film was determined using digital balance and the average weight of all fast dissolving films are given in table. All the batches were uniform in weight with no significant difference in the weight of the individual formulations from the average value. Weight variation was found to be in the range of 36 to 47 mg for prepared films. Among all batches F3, F4, F6 showed the highest weight of film may be due to increased concentration of polymer.
Moisture content
Table 8
Moisture content of different formulations of teneligliptin
|
Formulation code |
F1 |
F2 |
F3 |
F4 |
F5 |
F6 |
|
Moisture Content |
0.5 |
0.9 |
0.5 |
0.8 |
1.5 |
1.2 |
Percentage moisture content was calculated to check the integrity of films at the dry condition. Also, the mechanical properties of the films are greatly affected by the moisture content. This quality control parameter enables to detect the protection of the films against drying out during storage. All the formulations showed % moisture content in between 0.5% to 1.5% to retain the plasticizing effect of the films.
Folding endurance
Table 9
Folding endurance of different formulations of teneligliptin
|
Formulation code |
F1 |
F2 |
F3 |
F4 |
F5 |
F6 |
|
Folding Endurance |
118 |
113 |
105 |
109 |
148 |
135 |
The films were subjected to folding endurance to evaluate the flexibility studies and also it gives an indication of brittleness of film. The folding endurance of the fast-dissolving film was determined by repeatedly folding a small strip of the ODF at the same place till it broke and the average folding endurance of all fast-dissolving film is given in table.
Surface pH
Table 10
SurfacepH of different formulations of teneligliptin
|
Formulation code |
F1 |
F2 |
F3 |
F4 |
F5 |
F6 |
|
Surface Ph |
5.6 |
6.4 |
5.8 |
6.1 |
6.3 |
5.7 |
The surface pH of the ODFS was determined to investigate the possible side effects due to change in pH In-vivo, since an acidic or alkaline pH may irritate the oral mucosa. Surface pH was determined by using pH meter. The surface pH of formulated ODFS was found to be in the range of 5.5 to 6.5 which indicates that the formulated ODFS were in the neutral pH range and would not cause any irritation after placing in the oral cavity.
In-Vitro Disintegration Time
Table 11
Disintegration time of different formulations of teneligliptin
|
Formulation code |
F1 |
F2 |
F3 |
F4 |
F5 |
F6 |
|
Disintegration Time sec |
54 |
57 |
45 |
51 |
48 |
58 |
Film of 2×2cm2 size taken and disintegration time checked visually. In each case three fast dissolving films were used and average disintegration time was calculated, the results are shown in table
Drug content
Table 12
Determinationof drug content of different formulations of teneligliptin
|
Formulation code |
F1 |
F2 |
F3 |
F4 |
F5 |
F6 |
|
Drug content |
86.5 |
89.1 |
96.3 |
91.2 |
94 |
85.8 |
Table 13
In-vitro release study of different formulations of teneligliptin Fast dissolving film
Table 14
Regression coefficient (r) values of drug release data obtained from various kinetic models
The Prepared fast dissolving films are evaluated for drug content uniformity. Drug content test was performed to ensure uniform distribution of the drug. Drug content varies between all the films.
According to USP requirement, criteria for drug uniformity should be 85 %-115% of the label claim.
Kinetic study
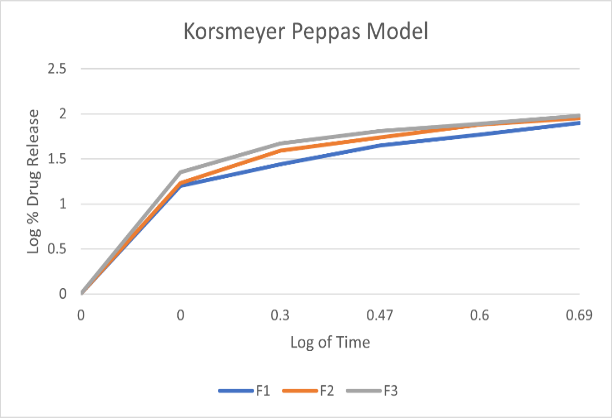
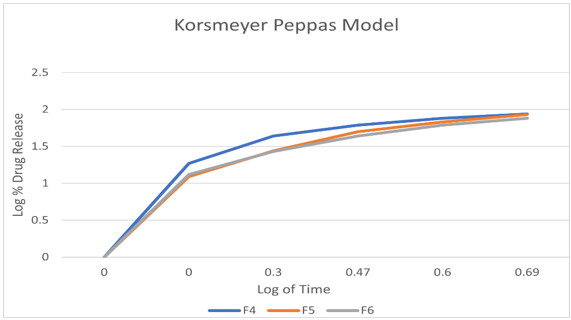
To investigate the mechanism of drug release from the teneligliptin mouth dissolving films various kinetics models like zero order, first order, Higuchi’s and Korsmeyer-Peppas equations were applied to the in vitro release data. The values of correlation-coefficient (r2) for all the selected formulations were high enough to evaluate the drug release behavior. The kinetic results revealed that the selected formulations followed Korsmeyer-Peppas as correlation-coefficient (r2) values ( 0.9920-0.9989) of Korsmeyer-Peppas are higher than that of first order values (0.9406-0.9709).When the data was plotted as per Higuchi kinetics, fairly linear plots were obtained with correlation coefficient values ranging from 0.8355-0.9341 for all the formulations. The drug release was proportional to square root of time indicating that the drug release from teneligliptin mouth dissolving films was diffusion controlled. The release data obtained were also put in Korsmeyer-Peppas l in order to find out n values, which describe the drug release mechanism. The r2 values of teneligliptin mouth dissolving films were found in the range of 0.9406-0.9709.,the mechanisum of drug release is non-fickian diffusion .since they fitted well with Korsmeyer-Peppas models. This indicate the drug release depend on swelling and diffusion mechanism of release.
Conclusion
The orally disintegrating film is an emerging oral dosage form, which is elegant, easily portable, and does not require water for swallowing.
The aim of the present research work was to formulate the oral disintegrating film and evaluate different formulations of fast dissolving films of antidiabetic drug teneligliptin to achieve faster drug release. This study shows that it is possible to formulate FDF of teneligliptin with the intention of obtaining better therapeutic efficiency with increasing bioavailability and improving patient compliance.
Around six formulations of teneligliptin were developed as fast dissolving films using various excipients .Films were found to be satisfactory when evaluated for thickness, Weight uniformity, in-vitro drug release, folding endurance, drug content and disintegration time. The surface Ph of all the films was found to be neutral. ODFS containing low amount of polymer and plasticizer i.e., formulation F3 had shown optimum disintegration and in vitro release, when compared with other formulation. So, it was concluded that F3 is the Optimized formulation of all the series of formulations. The optimized formulation (F3) shows highest percent drug release of 96.65% at the end of 5 min with fastest disintegration time of 45 sec.
Present study reveals that the formulated films showed satisfactory film parameters. It can be concluded that, Mouth dissolving film containing teneligliptin can be prepared by solvent casting method and formulated OFS release the drug into systemic circulation rapidly from the site of administration providing an alternative approach to treat diabetes so it can be conveniently administered in the form of films.