Introduction
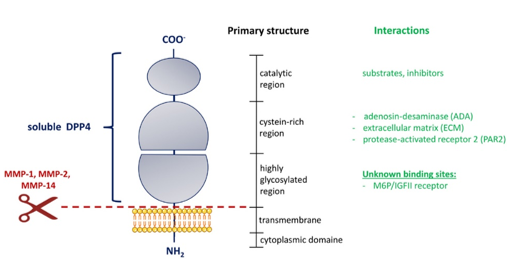
Enzyme Dipeptidyl peptidase 4 (DPP4) was first detected in 1966 as an aminopeptidase with the relatively distinctive ability to cleavea prolyl amide bond and was discovered by Hopsu-Havu and Glenner.
Dipeptidyl peptidase-4 (DPP4), also known as adenosine deaminase complexing protein 2 or CD26 (cluster of differentiation 26) is a protein that, in humans, is encoded by the DPP4 gene.DPP4 is related to FAP (Fibroblast activation protein alpha (FAP-alpha) also known as prolyl endopeptidase FAP), DPP8, and DPP9.1
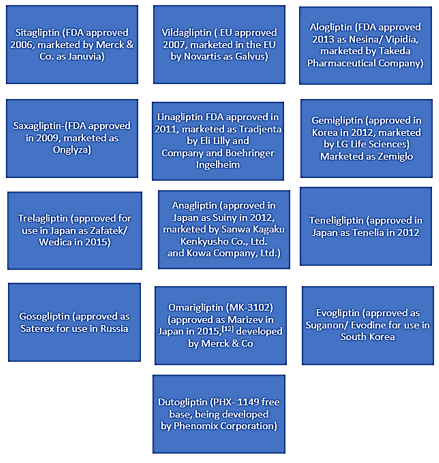
DPP4Is
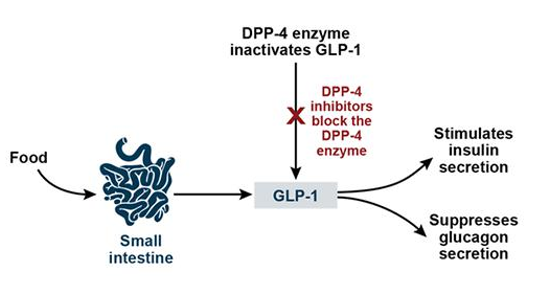
Dipeptidyl peptidase-4 inhibitors (DPP-4 inhibitors) are enzyme inhibitors that inhibit the enzyme dipeptidyl peptidase-4 (DPP-4). They are used in the treatment of type 2 diabetes mellitus (T2DM). Inhibition of the DPP-4 enzyme extends and improves the activity of incretins that play an important role in insulin secretion and blood glucose control regulation.2
Classification of DPP4Is
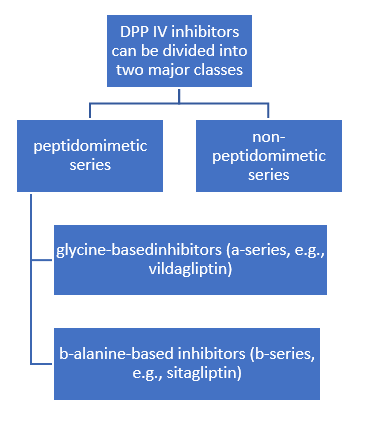
DPP4Is are also classified into two main classes, those that interact covalently with DPP-4 and those that do not. Also are classified either substrate-like or non-substrate-like. Substrate-like inhibitors are more common than the non-substrate-likes. They bind either covalently or non-covalently and have a basic structure where the P1-substituent occupies the S1-pocket and the P2-substituent occupies the S2-pocket.
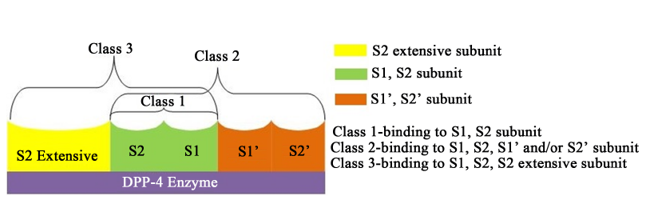
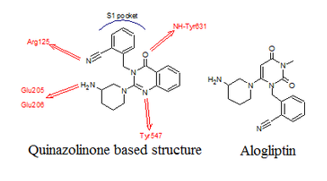
DPP-4 enzyme with binding sites
DPP-4 enzyme has various binding sites namely S1, S2,S1’, S2’ & S2 extensive subunit as shown in above Imagine. An interaction of DPP-4 inhibitors with S1 & S2 is consideredto be the basic interaction required for DPP-4 interaction. Further interaction with S1’, S2’ & S2extensive site may further increase the DPP-4 inhibition.3
Journey of DPP4Is
The first inhibitors were characterized in the late 1980s and 1990s. Each inhibitor was important to establish an early structure activity relationship (SAR) for subsequent investigation 2
The first DPP-4 inhibitors were reversible inhibitors and came with safety issues because of low selectivity. One of the first reported DPP-4 inhibitor was P32/98 from Merck. It used thiazolidide as the P1-substitute and was the first DPP-4 inhibitor that showed effects inboth animals and humans but it was not developed to a market drug due to safety concerned 3
Synthesis of Alogliptin
Alogliptin is a novel DPP-4 inhibitor developed by the Takeda Pharmaceutical Company.
A quinazolinone based structure would have the necessary groups to interact with the active site on the DPP-4 complex.
Quinazolinone based compounds interacted effectively with the DPP-4 complex and showed potent inhibition and excellent selectivity over related protease, DPP-8. However, short metabolic half-life due to oxidation of the A-ring phenyl group was problematic but suffered from low metabolic half-life.
It was found that when replacing the quinazolinone with a pyrimidinedione, the metabolic stability was increased, and the result was a potent, selective, bioavailable DPP-4 inhibitor named Alogliptin 4
At first, the efforts were put to make a fluorinated derivative. The derivative showed improved metabolic stability and excellent inhibition of the DPP-4 enzyme. However, it was also found to inhibit CYP 450 3A4 and block the hERG channel.
The solution to this problem was to replace the quinazolinone with other heterocycles, but the quinazolinone could be replaced without any loss of DPP-4 inhibition.
Alogliptin was discovered when quinazolinone was replaced with pyrimidinedione 5
Table 1
Summary of mean IC50 values of alogliption and sitaliptin for DPP4 and related serine peptidases
Results confirmed highly selective DPP4 inhibition by alogliptin, with no detected inhibition of DPP8 or DPP9 Alogliptin has shown excellent inhibition of DPP-4 and extraordinary selectivity, greater than 100000-fold over the closely related serine proteases DPP-8 and DPP-9. Also, it does not inhibit the CYP 450 enzymes nor block the hERG channel at concentration up to 30 µM. Based on this data, alogliptin was chosen for preclinical evaluation.
Animal / Pre-clinical studies
The pharmacokinetic parameters of alogliptin were evaluatedin three different animal models: rats (10 mg/kg oral), dogs(3 mg/kg oral) and monkeys (2, 10, 30 mg/kg oral). The PK profile of alogliptin was also studied after intravenous administration into rats, mice and monkey at 1 mg/kg.
In addition, in rat (data not shown), dog, and monkey treated with alogliptin, the plasma concentration oft he compound and the level of DPP-4 inhibition displayed agood correlation. Alogliptin also produced dose-dependent improvements in glucose tolerance and increased plasma insulin levels in female Wistar fatty rats. Following scale-up, GLP toxicology studies in rat and dog demonstrated the compound to be well tolerated.5
Interactions of alogliptin with other anti-diabetic agents (pioglitazone, voglibose, metformin and glibenclamide) were also examined on diabetic indices in animal models of diabetes. The effects of the combinations of the drugs were generally additive but the following synergistic effects were seen: alogliptin and pioglitazone mediated increases in pancreatic insulin content and the insulinogenic index in db/db mice; alogliptin and voglibose mediated increases in pancreatic insulin and plasma intact GLP-1 levels in db/db mice; alogliptin and metformin mediated increases in intact plasma GLP-1 levels and insulin secretion in Wistar fatty rats.
Overall, the PK profile in animals was qualitatively similar to that of humans. Alogliptin was readily and rapidly absorbed with a similar Tmaxin all species. Half-life values were similar in rats and dogs but longer in monkeys and humans. Plasma protein binding of alogliptin was low to moderate in all animal species and humans. Tissue distribution of alogliptin was wide but penetration into brain and spinal cord was very limited.
Pharmacokinetic drug interactions involving CYP enzymes are unlikely. Alogliptin had a low order of acute oral toxicity in rats and dogs.
Repeat-dose toxicity studies were performed in mice, rats and dogs. High relative exposures were achieved in these studies. The pivotal studies were of 6 months duration in rats and 9 months duration in dogs. No major organ toxicities were seen in this species or in mice.6
No skin lesions were seen in Cynomolgus monkeys treated for 13 weeks at doses resulting in exposures 27 times the clinical AUC. No major organ toxicities were observed with alogliptin in mice or dogs.
Repeat-dose toxicity studies of up to 13 weeks duration were conducted withalogliptin/pioglitazone and alogliptin/metformin combinations to rats. No new or exacerbated toxicities were noted with alogliptin/pioglitazone combinations. While no new toxicities were seen with alogliptin/metformin combinations.
The results were negative in all tests and alogliptin is unlikely to pose a mutagenic or clastogenic risk to humans.
No treatment related increase in tumour incidence was observed in mice or female rats in 2-year oral carcinogenicity studies Relative exposure at the NOEL was 27. Alogliptin is unlikely to pose a genotoxic or carcinogenic hazard to patients. Fertility was unaffected in rats at doses resulting in approximately 170 times the clinical AUC. No effects on the developing male reproductive system were seen in juvenile rat studies. Alogliptin was not phototoxic in hairless mice at high doses.
Primary pharmacology studies in animal models of T2DM support the use of Alogliptin for the proposed indication.
Conclusion
DPP-4 inhibitors are important oral antidiabetic agents that are slotted as second-line therapy after metformin failure as insulinotropic agents that have no intrinsic hypoglycaemia risk and are body weight neutral. Alogliptin has also demonstrated that in 15 RCTs to confer greater glucose-lowering efficacy in Asians than in non-Asians. The difference in the treatment response can be attributed to different BMI values, insulin action, and dietary habit, as well as genetic factors. Alogliptin was able to down-regulate atherogenic lipids such as log(TG)/HDL-C, T-C/HDL-C, or LDL-C/HDLC in addition to LDL-C, nonHDL-C or non-HDL-C/HDL-C . FDA has issued is warning that the type 2 diabetes medicines , gliptins may cause joint pain that can be severe and disabling. Currently Alogliptin was shown to reduce atherosclerosis, inflammation and coronary plaque.
Strengths of Alogliptin
Highly selective for DPP-4 than DPP-8 and DPP-9.
Can be given without due regard to meals.
Absolute Bioavailability of 100 %, so full drug is available for action
Fixed dose Combinations are available in developed countries such as Japan EU and US.
Promising HbA1c reduction in Asian population -0.75%.
Effective and safe in elderly patients.
The Alogliptin dosage does not need to be adjusted in patients with mild renal impairment.
Alogliptin is generally safe, with a low risk of hypoglycemia, weight gain, acute pancreatitis, and gastrointestinal adverse events.
ENDURE Trial has revealed that, Alogliptin 12.5 mg and 25 mg once daily was found to have sustained antihyperglycaemic efficacy over 2 years in patients with inadequate glycemic control of T2DM on Metformin with similar safety and tolerability profiles along with hypoglycemia, which occurred at a substantially higher rate with Glipizide.
EXAMINE trial (CVOT) clearly demonstrated that there was no indication that Alogliptin led to any increase in new hospital admissions for heart failure or worsened outcomes for patients with a previous history of heart failure.
In Asian patients, Alogliptin reported a higher efficacy and good tolerability, as compared to no-Asian patients with diabetes mellitus.
Multicentric, Phase 4, CONFIDENCE study concluded that Alogliptin and fixed dose combination of Alogliptin plus Metformin was found to be a promising option in management of Indian T2DM patients in relation to safety and efficacy.
Conflict of Interest
Dr. Abhijit Trailokya, Dr. Kavita Inamdar, Mr. Yogesh Bhide & Mr. Gopal Wawde are associated with Indoco Remedies Ltd.
Acknowledgment
We gratefully acknowledge the significant contributions of Mr. Gopal Wawde (General Manager, Formulation Development), Mr. Pramod Korde (Deputy Manager, Formulation Development), Mr. Amit Kale (Manager, Analytical Method Development.), Mr. Yogesh Mane (Manager, Analytical Method Development), Mr. Yogesh Bhide (Executive GM Formulation Development) & Dr. Kavita Inamdar (Chief Technical Officer R and D, Indoco Remedies Ltd.) in the development of Alogliptin and the fixed-dose combination of Alogliptin and Metformin for Indian Type-2 Diabetic Mellitus patients. We also acknowledge the sincere effort of Dr. Amar Shirsat, Dr. Shaijesh Wankhede & Mr. Avinash Talware who helped in referencing & preparing this text. Thanks to Dr Sunil Chaudhry for helping in editing the manuscript and his guidance for publication.