EV Nanoprobes Have the Ability to Supply Biomolecules
As stated in the Glossary, biologics have accounted for thirty percent of all FDA-approved drugs since 2014. By the year 2023, five of the fifty-five new medications were biosimilars. 15 biosimilars have been authorized by the FDA as of this moment. Out of the 39 new medications that were approved by the EMA in 2023, eight of them were biosimilars. The FDA has given its approval to eighty biosimilars since 2006. The approvals demonstrate an increase in the use of biologic treatments. The stability concerns of these drugs, which are caused by their susceptibility to fluctuations in temperature, solvent, and pH, as well as on-target off-tissue side effects, need to be addressed.1 Liposomes and other artificial nanoparticles have been used for transporting biologic medications for a long time.2, 3 Recently, however, there has been a resurgence in interest in nanomedicine as a more effective drug delivery vehicle for the treatment of a wide variety of disorders.4, 5 This is because to the exceptional targeting capabilities, improved safety profile, and high biocompatibility of extracellular vesicles. It is possible that extracellular vesicles are more effective at transporting medicine than nanoparticles.6 The fact that they are able to genetically change parental cells gives them the potential to manufacture and integrate therapeutic biomolecules into EVs using their own machinery. This is only one of the numerous benefits that they have over conventional drug delivery methods. When compared to external loading, endogenous loading can make the process of manufacturing, purifying, and storing biological drugs more straightforward7 (see Figure 1 BI). There is a possibility that EVs will enhance the therapeutic efficacy of new biologic medicines and help offset some of their disadvantages.In this research, we discuss the various endogenous ways for creating and loading biological materials into extracellular vesicles (EVs), as well as the existing strategies for loading extracellular vesicles (EVs). For the purpose of shedding light on the numerous EV-based therapies, we will examine the most prevalent methods to maximize EV cargo loading and the therapeutic applications of these methods.
Loading Instructions for Endogenous Extracellular Vesicles
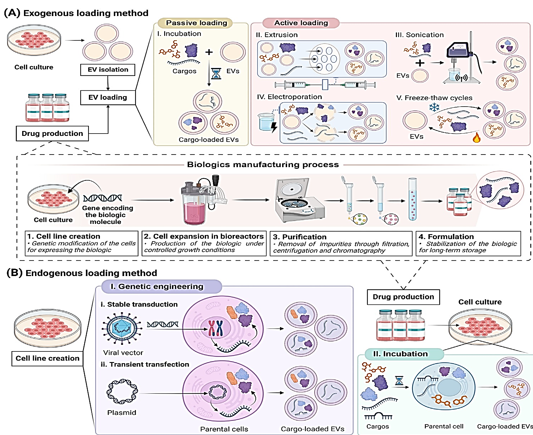
Extracellular vesicles can be loaded either from the outside or from the inside. The loading of synthetic nanoparticles into extracellular vesicles (EVs) has been accomplished by the use of exogenous methods, as demonstrated in Figure 1A. However, these approaches are detrimental to EVs because they modify the structure and membrane stability of the EVs (Table 1). It is possible for targeting and immunocompatibility to be compromised when necessary membrane proteins are broken down.8 Extracellular vesicles and the payloads they carry could be damaged by forces from the outside. 9, 10 Therefore, endogenous methodologies, which are not harmful to extracellular vesicles (EVs) and do not involve their destruction, have been widely used in research (Table 1).
At the time of EV biogenesis, the endogenous system, also known as the pre-encapsulation mechanism, loads cargo. It is possible to load cargo through endogenous incubation or genetic engineering. The incubation process comprises loading extracellular vectors (EVs) onto cargo and then adding it to the cell culture medium in a concentration gradient, as shown in Figure 1 BII. Because of this, parental cells are able to integrate cargo into their cytoplasm." Drugs that are loaded into incubation must not cause harm to the cells of the parents.11 The purpose of genetic engineering is to boost the expression of therapeutic biomolecules by modifying the DNA of the parent cells. The target molecule is synthesized by the body, and then it is encased in energetic vehicles (EVs),which is a novel approach.12 When it comes to loading extracellular vectors (EVs) with therapeutic macromolecules, genetic engineering is a more integrated and straightforward way than incubation and exogenous procedures. In this study, the term "endogenous method" is used exclusively to refer to the process of genetic engineering of parental cells. This method is the most efficient way to increase the loading efficiency of proteins and nucleic acids.13 Scientists loaded biologic pharmaceuticals onto EVs by the use of stable transduction and transient transfection of parental cells. Lentiviruses are utilized in the process of transduction in order to introduce the therapeutic payload gene into the DNA of the cell.14, 15 This can be seen in Figure 1 Bi. As demonstrated in Figure 1Bii, the process of transfection involves the introduction of therapeutic oligonucleotides or plasmids that can express the therapeutic gene into parental cells. Use is made of a number of different therapeutic oligonucleotides. mRNAs, circRNAs, long noncoding RNAs, and siRNAs are some examples. Depending on the gene that is being silenced, these RNAs can either temporarily or permanently silence the epigenetic expression of the gene. 16 EVs can also be produced by transformed cells using the chemical that is produced by the overexpressed gene. Because of this, extracellular vesicles are able to transport growth factors and cytokines.17, 18, 19, 20, 21 Numerous specialists have demonstrated that genetically produced extracellular vesicles are able to maintain their physicochemical qualities more effectively than exogenous loading methods (Table 1). For the purpose of treating lung injury, Salazar-Puerta and colleagues modified dermal fibroblasts to secrete extracellular vesicles (EVs) that expressed the anti-inflammatory cytokines IL-4 and IL-10. The genetic engineering loading did not cause any damage to the EV lipid membrane, according to the findings of these researchers.21 Lu and colleagues developed a therapy for skin lesions that is based on genetic engineering. A cell line with robust NF-κB siRNA secretion was created by researchers by the use of lentiviral transduction of mesenchymal stem cells (MSCs) derived from adipose tissue. At the same time, non-modified cells served as controls. The size and expression of membrane indicators were identical in exosomes from control cells loaded with siRNA and those without.22
Table 1
Comparison of exogenous loading and genetic engineering in EV cargo loading
Enhancing EV Biologic Drug Loading Via Engineering
It is necessary to have an understanding of the complex cargo integration processes in order to load biologics into endogenous vehicles. The cargo sorting process for EV production involves a number of molecules and is subject to stringent supervision. 23 Recent advancements in molecular biology, mass spectrometry, and next-generation sequencing have led to the discovery of molecules that are responsible for the selection and loading of extracellular vesicles cargo. 24 Having this insight has resulted in the development of novel ways for loading EV biologics. 25 These forward-thinking technologies include molecular sorting modules, which are responsible for adding EV payload. Scientists have the ability to genetically modify parent cells using MSM in order to increase the expression of biologic drugs. The conversion of biologics into EVs occurs naturally during biogenesis. 26
Figure 1
Delivery of drugs laden with EV. An illustration of the exogenous loading. By using this method, EVs are separated out and biologic medicine is produced (1–4). Biologic medicine is contained within EVs. By using this procedure, tiny synthetic medicines are loaded. An exogenous drug loading that is either passive or active. The process of incubating the drug with EVs is an example of passive loading. Active loading methods include extrusion, sonication, electroporation, and freeze-thaw cycles. The endogenous loading graph is shown here. The practice of incubation and genetic engineering is widespread. Transfection, either continuous or transient, can be used to affect the genetic makeup of parental cells. By combining the synthesis of biologic drugs with the loading of EVs, genetic engineering can improve efficiency. Following the establishment of the cell line, more optimization can be performed to integrate the synthesis and separation of EV with the formulation and purification of biological medications. This ensures that essential vesicles are produced under specific conditions and that they are kept apart from biologic medicines. Through the process of generating and adding biological medicines (1-4) to cell culture, incubation develops various phases, similar as when exogenous procedures are conducted. EV protein medication loading has seen recent advancements, as shown in this figure from BioRender.
.
Methods developed recently to improve EV protein medication loading
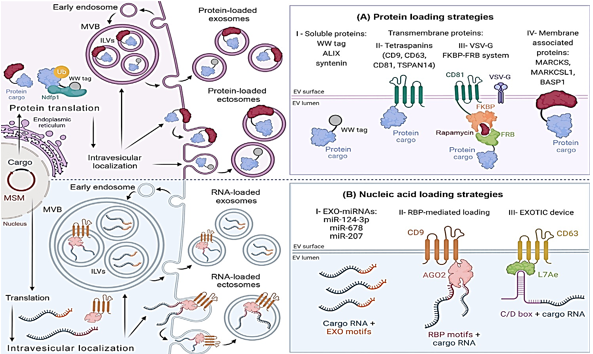
Improvements in protein cargo loading have been made possible by a variety of methods that imitate EV protein loading systems. By recognizing and encapsulating ubiquitin-tagged proteins into extracellular vesicles, the late-domain route, also known as the L-domain pathway, is responsible. In order to carry out this plan, the NEDD4 ubiquitin ligase family engages in a conversation with NDFIP1. According to the findings of the research, WW tags are attached to target proteins by the L-domain motifs of NDFIP1. Protein motifs known as WW tags consist of two tryptophan (W) residues that are next to one another. NEDD4 is able to ubiquitinate and integrate into exomes with greater ease when the cargo protein in question has a WW tag.27 NDFIP1 is responsible for the packaging of WWTR1, also known as TAZ, into exosomes, as Cheng et al did. It is demonstrated in Figure 2AI that NDFIP1 is the only one that can identify TAZ with its WW domain intact, in contrast to cTAZ, which is a shortened TAZ that does not contain it. Intriguingly, this demonstrates that NDFIP1 only interacts with TAZ and not with cTAZ. Tetraspanin-rich microdomains are responsible for loading extracellular vesicles with proteins. In order to develop an effective medium for the transport of extracellular vectors (EVs), Zuppone et al. genetically incorporated an RFP reporter protein onto CD9, CD63, and CD81 tetraspanins. CD9-RFP was selected as the most effective recombinant protein due to its ability to be delivered to recipient cells and extensively sorted onto extracellular vectors (EVs). Using an EV MSM that has been folded and sorted can help prevent lysosomal degradation28 There is also a significant load of tetraspanin TSPAN14. In contrast to CD63 and other EV biogenesis proteins such as ALIX and syntenin, which can only load between 40 and 70 EGFP/vesicles, it was able to load 150 EGFP/vesicles.29, 30 The efficiency with which cargo is loaded is impacted by cargo attributes, which makes it challenging to select the appropriate MSM. These procedures are straightforward to adopt and make advantage of biological mechanisms to prevent injury to parent cells. Additionally, the vesicular stomatitis virus G is capable of loading EVs. Multiple studies have demonstrated that VSVG is capable of loading extracellular vesicles with a wide variety of chemicals. 31 Fusing EV membranes is one of the ways that VSVG helps to increase cell internalization and cargo release.32 The endosomal escape pathway is a barrier to the EV-mediated intracellular administration of medicines. There have been several studies33 that have shown that proteins that are given in this manner prevent the lysosomal breakdown of recipient cells.
VSVG-based synthetic EVs and rapamycin-regulated protein-protein controlled loading were both improved by Somiya and colleagues over the course of their research. FK506-binding protein (FKBP12) and the FRB domain are both complexed by rapamycin, which is a relatively tiny chemical. Somiya and his colleagues fused the target protein with FRB after first combining CD81 tetraspanin and FKBP from the previous step. Rapamycin improved the interaction between FKBP12 and FRB, which resulted in cargo being recruited to EVs (Figure 2 AIII). The researchers believe that the viral origins of VSVG could potentially elicit immunological reactions, which would render it unsuitable for injection into living organisms. Therefore, proteins that are not viral are advised for use in treatment.34 It has been demonstrated by Ilahibaks et al. that this method is capable of transporting intracellular protein in vivo, despite the constraints that were anticipated.35 FRB/FKBP heterodimerization and syncytin-1, a naturally occurring human protein, were utilized by Bui et al. in the development of a method that is analogous to the one described above. Within the laboratory, this alternate fusogen is able to enhance both VSVG and cargo movement. 36 In order to enhance protein loading, therapeutic compounds can be combined with membrane-associated proteins that are created by electronic vehicles. Proteomic study led to the discovery of the MARCKS protein family, which includes MARCKS, MARKCSL1, and BASP137 (Figure 2AIV). This family has potential advantages. Research has shown that extracellular vesicles (EVs) are involved in a number of biological functions; however, it is not understood what role they play in the process of biogenesis. They change the dynamics of the actin cytoskeleton as well as the trafficking of vesicles 38.
Figure 2
Endogenousextracellular vesicles, therapeutic cargo loading can be accomplished. Parentalcells are genetically modified to encode the therapeutic payload as well as amolecular sorting module (MSM) in order to improve the loading of the cargo. Biogenesis is the process via which therapeutic cargo becomes EVs. The loading of cargo into ectosomes or exosomes is determined by biogenesis. Through theprocess of plasma membrane evagination into extracellular space, ectosomes are formed. After invagination of the plasma membrane by endosomes, exosomes areformed. The early endosomes develop into the later endosomes. There is adevelopment of the endosomal membrane folds as well as intraluminal vesicles. There are ILVs present in multi vesicular body lumens. During the process ofreleasing ILV exosomes, MVBs combine with plasma membranes. The EV proteinloading approach is the first option. Encapsulation of EV nucleic acid issecond. Exosomal microRNA, also known as EXO-miRNA; NEDD4 family interacting protein 1, also known as NDFIP1; RNA-binding protein, also known as RBP;ubiquitin, also known as Ub; and vesicular stomatitis virus, also known asVSG-G. This figure was generated using BioRender.
The most recent techniques for enhancing the drug loading of EV nucleic acid
In addition, the intricate processes that control the loading of proteins and nucleic acids into extracellular vesicles are connected. Even while the composition of EVs is mostly determined by their mother cell, there are a variety of subtypes. 39 Exosomes have a greater number of microRNAs, according to a number of studies, while ectosomes have a genetic composition that is comparable to the transcriptome of the parent cell. MiR-124-3p, miR-678, and miR-207 have all been found to be expressed in exosomes, according to recent research. A higher level of miR-343 and miR-140-3p expression is seen in parental cells (Figure 2 BI). Because of the unique patterns that miRNAs exhibit, miRNA loading is dependent on the sequence of DNA.40, 41
RBPs, like as hnRNPs, have the ability to load EVs with nucleic acids. Exosome miRNA trafficking is regulated by hnRNPA2B1, which is one of the hnRNPs that has received the most investigation. For the purpose of gene therapy, this chemical has the potential to load EXO-motifs miRNAs into the lumens of exosomes. 42 Research indicates that hnRNPA2B1 is responsible for modulating the packing of circRNA. 43 Searching for RBPs that target the inclusion of EV RNA is extremely important. Numerous investigations have uncovered more RBPs that are capable of accomplishing this. SYNCRIP, AGO2, VAP-A, YBX-1, FMR1, and HuR are some of the examples of these. Es-Haghi and colleagues derived hCD9.hAGO2 from their own personal experiences. Exosome biogenesis-related CD9 and RBP AGO2 are elements that are contained inside this vector (Figure 2BII). EVs that were transformed with the hCD9.hAGO2 vector were shown to contain higher quantities of miRNA compared to cells that overexpress miRNA, according to the findings of the study.44 There is a greater abundance of the zipcode, which is a component of the 3′-untranslated region (UTR) of mRNA, in EVs.45 Additionally, the 3'-untranslated region (UTR) of the mRNA of interest can be modified to include a sequence that is similar to a Zipcode. The loading of EV mRNA is improved by EXOTIC. Additionally, the rRNA packaging protein L7Ae and the C terminus of CD63 are utilized in this technique. A 3'-UTR C/D box is the mechanism by which it binds therapeutic mRNA (Figure 2BIII).
Table 2
Therapeutic applications of genetically engineered evs for delivering biologic drugs
[i] Abbreviations: ACE2, angiotensin-converting enzyme 2; ADMSCs, adipose mesenchymal stem cells; ADSCs, adipose-derived stem cells; BDNF, a factor derived from the brain; Bmp2, a protein involved in bone development; BMSCs, stem cells derived from bone marrow; COL1A1, a type of collagen found in the extracellular matrix; CRC, also known as colorectal cancer; CTGF, also known as connective tissue growth factor; DMD, also known as Duchenne muscular dystrophy, DNMT1 is the DNA methyltransferase 1, while DTA refers to the catalytic A domain of diphtheria toxin. EDNRA stands for endothelin A receptor, while EGF represents epidermal growth factor. EGFR, on the other hand, stands for epidermal growth factor receptor. EMT refers to the process of epithelial-to-mesenchymal transition, and eNOS is the endothelial nitric oxide synthase. FOXF1, forkhead box F1; FOXO1, forkhead box transcription factor O1; GDNF, glial cell line-derived neurotrophic factor; GPX4, glutathione peroxidase 4; GSDMD-N, gasdermin D N-ter- minal fragment; GSTP1, glutathione S-transferase P1; HDAC4, histone deacetylase 4; HEK, human embryonic kidney; HGF, hepatocyte growth factor; HIV-1, human immunode- ficiency virus 1; HO-1, heme oxygenase 1; HOTAIR, lncRNA HOX transcript antisense RNA; HPMCs, human omentum tissue-derived mesothelial cells; HTT, Huntingtin; hucMSCs, human umbilical cord mesenchymal stem cells; hUSCs, human urine-derived stem cells; iPS, induced pluripotent stem cell; IRAK 1, interleukin-1 receptor-associated kinase 1; ITGA6, integrin subunit α6; JAZF1, zinc-finger gene 1; LDLR, low-density lipoprotein receptor; MAPK1, mitogen-activated protein kinase 1; MMPs, matrix metalloproteinases; MSCs, mesenchymal stem cells; NP, nucleus pulposus; NPC, nasopharyngeal carcinoma; NRF2, nuclear factor erythroid 2-related factor 2; NSCs, neural stem cells; PEDF, pig- ment epithelium-derived factor; PFKFB3, 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3; PMSCs, placental mesenchymal stem cells; polyQ, polyglutamine; PTPN9, protein tyrosine phosphatase non-receptor type 9; RNP, ribonucleoprotein; ROS, reactive oxygen species; SIRT1, sirtuin 1; SMAD3/5, SMAD family members 3 and 5; SOCS2, suppressor of cytokine signaling 2; SOX11, SRY box transcription factor 11; TAF1, TATA box-binding protein-associated factor 1; TECs, tubular epithelial cells; TGF-β1, transforming growth factor β1; TPP1, tripeptidyl peptidase 1; TYMS, thymidylate synthase; USP2, ubiquitin carboxyl-terminal hydrolase 2; VEGF, vascular endothelial growth factor; VEGFA, vascular endothelial growth factor A; ZPAMt, zinc-finger protein fused to PWWP, ADD, and methyltransferase.
In some cases, exoTIC devices may contain CX43 protein. Therapeutic messenger RNA is transported to the cytoplasm of the target cell by CX43.85, 83
Treatment using EVs loaded with GMOs.
The ability of genetically engineered extracellular vectors (EVs) to transfer biomolecules into specific cells for therapeutic purposes has been demonstrated by a number of research.52 There is considerable potential for the in vivo translation of in vitro discoveries.63, 79 The preclinical models of genetically designed loaded EVs that carry biologics for the treatment of cancer, cardiovascular disease, and disorders of the nervous system show promise. 46, 53, 61 As shown in. Table 2
The selection of an EV cell source is an essential step in the creation of EV pharmaceuticals. Evolved cells (EVs) usually maintain the traits and biological functions of their parent cells.86 EVs produced from MSCs increase the communication between immune cells and the development of blood vessels. However, EVs that are produced from immune cells carry MHC molecules, which have the ability to either stimulate or repress immunological responses. EVs that are produced from tumors are able to target particular body regions and encourage the development of tumors.87, 88, 89 The biological features of each and every EV-producing cell need to be assessed in order to provide a safe and effective treatment.
Genetically Modified Extracellular Vesicles for Various Diseases
Transportation vehicles that Have been genetically modified for the purpose of treating cardiovascular disease
Cardiovascular diseases pose a threat to the health of people all over the world and call for the development of novel treatments. Recent research has investigated the possibility of using genetically modified loaded extracellular vesicles (EVs) as a treatment for cardiovascular disorders. Contrary to conventional medical practices, therapies based on nucleic acid have the potential to cure or reduce symptoms. This can be accomplished through the regulation of gene expression as well as intracellular signaling pathways.1 2. An investigation into the protective effects of miR-183-5p-overexpressing MSC-derived exosomes against myocardial ischemia/reperfusion was carried out by Mao et al. The microRNA MiR-183-5p was able to permeate cardiomyocytes, hence reducing oxidative stress and mortality in cardiomyocytes that had been separated or decreased. 46 This resulted in a considerable improvement in cardiac function. A similar experiment was carried out by Liu and colleagues in order to develop an AMI treatment. After that, the researchers investigated the inflammatory environment of AMI as well as the molecular mechanisms involved in heart remodeling. AMI mice exhibited a decrease in the expression of miR-302d-3p. It was discovered in the study that EVs loaded with miR-302d-3p improved the health of the heart in AMI mice. Significant reductions were observed in the infarcted area, myocardial fibrosis, inflammation, apoptosis, and cardiac dysfunction. MiR-302d-3p was loaded into MSC-derived extracellular vesicles using their endogenous mechanism.47 There have been recent developments that point to heart-specific nucleic acid therapies.
EVs that fight cancer have been genetically engineered
Both treatment resistance and metastasis are factors that influence cancer mortality rates internationally. A number of studies have demonstrated that extracellular vesicles are capable of transporting messenger RNAs, microRNAs, circRNAs, and proteins. According to the findings of these investigations.59, 19, 53, 60 EVs have the potential to be an effective and selective cancer treatment.Figure 2 is the reference.
The synthesis of the diphtheria toxin (DTA) catalytic A domain was increased through the use of genetic modification of parental cells by Dancourt et al. Because it prevents the production of proteins, this toxin is lethal to cancer cells. The cell cannot be reached by DTA on its own, even when it is present at large concentrations in the extracellular media. On the other hand, DTA in the cytoplasm is lethal to cells. EVs were shown to be capable of transporting DTA into the cytoplasm of recipient cells by the authors. The creation of proteins was inhibited, and cancer cells were eliminated as a result. 52 In order to investigate the efficacy of DTA for tumor ablation, researchers have utilized other carriers such as viruses and liposomes.90 On the other hand, problems with manufacturing, formulation, and immunogenicity have held down the development process. It is easier to create and produce extracellular vesicles DTAs when endogenous loading is used. Since EVs transport poisons in a more natural manner than liposomes or viruses, they are less likely to be rejected. 52 Xiao et al. conducted a separate experiment in which they found that genetically altered loaded EVs were effective in treating colorectal cancer that was resistant to oxaliplatin. While the expression of miR-1915-3p was much higher in oxaliplatin-resistant cell lines, it was significantly lower in non-tumorigenic intestinal cells. The therapeutic miRNA was transmitted to a colorectal cancer cell line by first being overexpressed in a nontumorigenic intestinal cell line and then being transferred to the colorectal cancer cell line for use. MicroRNA-1915-3p was found to reduce drug-resistant cancer-causing genes in mice xenograft models, which resulted in an increase in oxaliplatin sensitivity. 53 Pang et al. conducted research on 5-fluorouracil (5-FU) chemotherapy with the goal of enhancing the treatment of colorectal cancer. They developed colorectal cancer tumor cell lines that overexpress miR-323a-3p for the reason that tumor-derived exosomes are attracted to the cells from which they originated. Exosomes that carry miR-323a-3p preferentially target tumors, hence lowering growth and enhancing 5-FU tumor killing. 55 Finding a safe cancer treatment that decreases side effects from chemotherapy and radiotherapy is another important challenge. The results verified a commonly held opinion that has been generally recognized for a long time. For the purpose of addressing this matter, Guo et al. investigated the impact that exosomes carrying circDIDO1 have on the progression of gastric cancer. The therapeutic circRNA was successfully carried to the cancer cells in the stomach by the altered exosomes. As a result, the circRNA was able to absorb miR-1307-3p, which led to a rise in SOCS2 expression.
The cytokine-induced signal transduction that is inhibited by SOCS2 results in a reduction in the proliferation, migration, and invasion of tumor cells. Exosomes loaded with circDIDO1 were administered to mice, and histological examinations revealed no abnormalities or lesions in any of the vital organs, including the kidney, spleen, heart, liver, or lungs. This provides evidence that exosomes have the potential to be successful therapies for stomach cancer.59 EVs that have been engineered can also transport mRNA, miRNAs, and circRNAs for the therapy of cancer. mRNA, in contrast to other nucleic acids, communicates genetic information from DNA to ribosomes, which are responsible for the production of proteins.16 However, there is a substantial possibility that EV-delivered mRNA might be used to precisely regulate the production of proteins in particular cells. Researchers Xing et al. used extracellular vectors (EVs) to deliver GSDMD-N mRNA to cancer cells. The GSDMD-N protein was produced as a result of the translation of the mRNA payload in the target cells. Because of pyroptosis, the tumor expanded at a slower rate. GSDMD-N administration through endogenous loading is associated with a number of disadvantages. It should be noted that parental cells may go through pyroptosis prior to the translation of mRNA and the release of EV. In order to find a solution to this issue, puromycin was utilized to inhibit the translation of parental cell mRNA. By using this approach, cells were preserved, and EVs were able to encapsulate mRNA. EV sorting is prevented when mRNA is translated correctly in parent cells because it binds to ribosomes and prevents it from moving around. The increased incidence of noncoding RNA in EVs can be explained by this strategy. Innovative technique developed by Xing and colleagues for encapsulating messenger RNA (mRNA) in extracellular vesicles(EVs) contributed to the advancement of scientific knowledge, 60 GM-loaded EVs for the treatment of disorders of the nervous system
EVs are fascinating as potential therapies for neurological problems
because of their immunological privilege and their ability to traverse the blood-brain barrier.91 As a result of recent research, it has been demonstrated that endogenously loaded EVs are capable of transporting big proteins and treating neurons. Zhou et al. took MSCs and collected their extracellular vesicles (EVs) in one of their studies. In this study, a novel therapy for cerebral ischemia was studied. It is common knowledge that BDNF possesses effects that are neuroprotective. Because it is unable to pass through the blood-brain barrier, the high-level chemical molecule known as BDNF degrades very quickly. Given this, clinical translation necessitates the implementation of an efficient BDNF delivery mechanism. MSCs have the ability to regulate the immune system. The particular characteristics of the EVs may have significant effects in the afflicted areas. Zhou et al. discovered that intranasally administered extracellular vectors (EVs) targeted the peri-infarct region of an ischemic stroke mouse model due to the attraction of chemokines and the permeability of the brain. Increases in neurogenesis, angiogenesis, synaptic plasticity, and fiber preservation were observed as a result of focused administration.18 miRNAs and other biological molecules have the ability to deactivate the pathways that are responsible for neuronal death to occur. The researchers Shen et al. evaluated the potential of miR-410 to treat hypoxic/ischemic brain injury (HIBD) by inserting it into bone marrow-derived mesenchymal stem cells (MSCs). The researchers discovered that separated EVs were able to treat primary neurons in vitro by targeting histone deacetylase and deactivating the WNT pathway, which is responsible for the death of neuronal cells. When EVs loaded with mi-R410 were administered to HIBD mice, cognitive performance was likewise improved.63
EVs that have been loaded with genetically modified DNA have been the subject of extensive research because of their potential to improve treatment for a wide variety of common diseases. Among these are issues pertaining to the respiratory system, the kidneys, the musculoskeletal system, dermatology, inflammation, bacteria, and metabolism. Table 2 provides a summary of these investigations.21, 17, 18, 22, 23, 24, 25, 26, 27, 92, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 93, 94, 95, 96, 97, 98, 99, 100, 44, 45, 85, 83, 52, 63, 79, 46, 53, 61, 86, 87, 88, 89, 47, 60, 90, 55, 91, 69, 81, 84 which can be found here.MSCs are preferred for EV generation due to their regenerative, anti-inflammatory, and immunoregulatory capabilities. Inflammatory illnesses include fibrosis, IBD, and osteoarthritis benefit from these features. 17, 81, 72 Several teams have added biologic drugs to MSC-derived EVs to boost their therapeutic potential. Researchers found that EVs supplemented with glial cell line-derived neurotrophic factor (GDNF) increased MSCs' renal fibrosis-reduction effects. They did this by increasing angiogenesis and reducing fibrosis. 17 EVs supplied with IL-27 from parental MSCs via lentivirus transduction improve intestinal epithelial barrier integrity and diminish neutrophil activity in IBD mice models 81. MSC-derived EVs are angiogenic and aid wound healing. EVs can be loaded with molecules to improve vascularization.77, 78
Since macrophages regulate immunity and inflammation, they have also been used to generate EVs. Membrane proteins on macrophage-derived EVs can lead them to inflammation. 101 Tang et al. found that EVs loaded with IL-10 from genetically engineered macrophages targeted the injured kidney in a mouse model of ischemia acute renal injury. This tailored administration reduced renal tubular damage, inflammation, and chronic kidney disease progression. 20 Additional study has examined using macrophage-derived EVs to treat inflammatory disorders. According to Guo et al., miR370-3p-loaded EVs showed promising outcomes in decreasing inflammation and boosting cell survival and proliferation in an IL-1β-induced chondrocyte model Osteoarthritis treatment may be possible with this discovery. 71 Last, EVs can convey the CRISPR/Cas9 technology to cure genetic diseases. This method uses a Cas9 protein and a guide RNA to edit DNA accurately to correct genetic mutations. The lack of secure, precise, and efficient delivery techniques makes EV use similar to CRISPR/Cas9 in clinical contexts. Recent studies show that EVs may carry and administer CRISPR/Cas9 DNA, RNA, and RNP for gene editing in vitro and in vivo. 102 RNPs are favored for endogenous loading because they avoid transcription and translation. This has made gene editing faster, more efficient, and less prone to have off-target consequences. 103 As indicated, scientists are targeting modified RNPs and EVs to increase RNPs.37 In a recent work, Yao et al. examined the in vivo activity of genetically altered loaded EVs that delivered RNPs targeting exon 53 of the DMD gene. Human DMD gene mutation in exon 53 mice received RNP-loaded EVs, which decreased skeletal muscle dystrophin synthesis. The study found that RNP-loaded EVs can change genes and increase dystrophin expression. 76 This novel method shows how EVs can transport the CRISPR/Cas9 system for precise DNA editing, especially for hereditary diseases.
Conclusion
There are many reasons EVs are good for transporting large macromolecules like proteins and genetic material. Several of these compounds have been loaded onto EVs and delivered to particular cells, demonstrating their therapeutic potential in lab and live tests. The preclinical investigations have not been tested in humans. Therefore, certain obstacles must be addressed to facilitate drug delivery clinical development. The variability of EV content, the lack of standardized production and isolation procedures, and the difficulties in scaling up production stand out. This review highlights genetic engineering as a strong tool for turning parental cells into efficient manufacturers producing stable and consistent extracellular vesicles. EVs can also be loaded with therapeutic proteins and nucleic acids using this method. This loading method enhances EV batch uniformity, surface structure, and time and effort over the exogenous method. In addition, parental gene engineering-based loading techniques have opened up new ways to solve biological therapy difficulties. Overall, the findings examined will influence the development of new medications. While many present bioengineering technologies concentrate single-step procedures, it is obvious that synergistic cargo loading and delivery approaches will substantially increase EV functionalization in the future. EV biology, notably biogenesis, systemic dispersion, and cell transit, must be understood. This understanding is important to maximize their therapeutic potential as future nanodelivery technologies.
