Introduction
Solubility is an essential element in achieving the required drug concentration in the systemic circulation for the desired pharmacological effect.1 Pharmaceutical researchers are always attempting to increase the solubility of weakly water-soluble drugs using innovative drug delivery platforms. Because many new chemical entities are impacted during the development stages due to solubility characteristics, these innovative drug delivery platforms demonstrated a potential role in improving solubility and thus bioavailability, increased residence within the biological system, site-specific release, and decreased toxicity.2, 3, 4
Pharmaceutical researchers are always exploring new drug delivery platforms to increase the solubility of medications that are not particularly well soluble in water. These innovative drug delivery systems demonstrate promise for enhancing solubility, bioavailability, residence in the biological system, targeting to site-specific release and toxicity, all of which are important during the development stages of many new chemical entities that are affected by their solubility characteristics. This innovative medication delivery technology may, in a nutshell, enhance treatment outcomes. Chemical modification, pH adjustment, micellar solubilization, solvency and solid dispersion are some methods for enhancing solubility; however, formulation techniques based on nanotechnology have shown exceptional efficacy in drug delivery. With their exceptional ability to encapsulate medicinal compounds inside their dendritic structure and attach them peripherally, these hyper-branched, mono-dispersed molecules are unique. Research has shown that dendrimers may increase the solubility of medications that are not very water-soluble. A few dendritic polymers have been designed, developed, and evaluated for application in medication delivery and product development because of these promising discoveries.5, 6, 7, 8
By increasing solubility, nanostructured dendrimers show significant potential for the effective delivery of antihypertensive drugs. Dendrimers are capable of greatly increasing the solubility of carvedilol, furosemide, and nimodipine. Dendrimers are classified as macro molecules because of their three-dimensional branching structure and the many spots where other molecules may get entangled. This is why studies involving host-guest chemistry are now trending. A new kind of polymer, dendrimers have earned the moniker "polymers of the 21st century.9, 10 " Because dendrimers' surface, interior, and core may all be tailored for multiple roles, there are more than fifty distinct dendrimer families, each with its own unique set of characteristics. For a long time, dendrimer chemistry has been both an academic hotspot and a formidable obstacle. One molecular unit makes up the dendrimer.11, 12, 13, 14
Both diuretics and beta-blockers have been used to treat essential hypertension for more than 3 decades. There are currently three generations of beta-blockers being used to treat heart failure. A member of the third generation of -blockers is carvedilol. Compared to other -blockers, carvedilol has a substantially higher amount of adrenergic inhibition, as well as significant vasodilating and antioxidative properties. Carvedilol has substantial interpatient variability and a low oral bioavailability (25–35%) despite these pharmacological advantages.15 Since the 1980s, nimodipine ((1,4) Dihydro(2,6)dimethyl(4-(3-nitrophenyl)(3,5)pyridine dicarboxylic acid 2-methoxyethyl1-methyl-ethyl ester) a typical L-type calcium channel blocker, has been utilized in a variety of settings. Nimodipine (NIMO) is widely used to treat cerebral vasospasm in patients with aneurysmal subarachnoid hemorrhage, trials exploring its antihypertensive efficacy after intravenous injection in subjects with intracerebral hemorrhage (ICH) are scarce. The (BCS) categorization places nimodipine in class II. primarily has a poor water solubility but has good permeability property.16, 17 Furosemide (Lasix) is a loop diuretic medication that is extensively used to treat hypertension. It is a medication of BCS class IV therefor Its oral bioavailability is extremely poor due to its low solubility and permeability.18, 19
Dendrimer-based nanocarrier systems frequently enter the bloodstream and interact with blood cells such as leukocytes and RBCs, the blood toxicity and biological characteristics of the dendrimer should be thoroughly investigated before systemic administration. In this study, we have investigated the reported dendrimer’s hemolysis study and cytotoxicity assays which is performed on the A-549 lung cancer cell lines. Reported nanostructured dendrimer (TG1.0, TG2.0, TG3.0) are used to improve the solubility of carvedilol (CAR) furosemide (FURO) and nimodipine (NIMO) by using higuchi and conner method. Effect of dendrimer generation and concentration on the solubility enhancement of carvedilol (CAR) furosemide (FURO) and nimodipine (NIMO) were studied. The experimental results showed that the dendrimer concentration and generation increased with increasing solubility of the hypertensive drug. The complex formation was confirmed by the infrared spectroscopy.
Experimental
Materials
API was generously provided by aim chemicals, Vapi, Gujarat. Phosphate Buffer Saline (PBS) was purchased from himedia labs (India). Blood sample for the haemolysis assay was collected from healthy Sprague-Dawley rat through retro-orbital route. Triazine trichloride, thiocarbamide, dichloromethane, methanol, and Acetone. All the reagent and solvent used for the synthesis of nanostructured dendrimer and their applications.
Synthesis of dendritic macromolecules
The following procedure was used to create a hydroxy-terminated dendritic macromolecules. At 0-5 ºC, triazine trichloride (0.02 mmol) interacted with thiocarbamide (0.01 mmol) to yield N,N'-bis(4,6-dichloro-1,3,5-triazin-2-yl)thiocarbamide as the core for dendrimer production. After washing with Acetone and Methanol, N,N'-bis(4,6-dichloro-1,3,5-triazin-2-yl)thiocarbamide was purified. The reaction of N,N'-bis(4,6-dichloro-1,3,5-triazin-2-yl)thiocarbamide (0.01 mmol) and diethanolamine (0.04 mmol) yielded hydroxyl terminated generation 1 (TG1) dendrimer. Washing and dispersing TG1 dendrimer in dichloromethane (DCM). TG1 dendrimer (0.01 mmol) was reacted with triazine trichloride (0.08 mmol) at 0-5 ºC to generate chlorine terminated half generation TG1.5 dendrimer in the same manner as in the first stage. In a similar manner to the second stage, chlorine terminated half generation dendrimer (TG1.5) (0.01 mmol) was reacted with diethanolamine (0.16 mmol) to produce full generation hydroxyl terminated dendrimer (TG2). The above two procedures were repeated to produce half-generation TG2.5 and full-generation TG3 dendrimers. FT-IR, 1H-NMR, 13C-NMR, and ESI-Mass Spectrometry were used to completely characterise the synthesised dendrimer.20
Estimation of λmax
The maximum wavelength (λ max) at which an active pharmaceutical ingredient (API) absorbs the greatest amount of light is called that wavelength. In order to determine the λ max of carvedilol, furosemide, and nimodipine, the active pharmaceutical ingredient (API) was first precisely weighed at 10 mg and then transferred to a 100-ml volumetric flask. The API was dissolved, and the volume was increased to the mark using methanol to produce solutions containing 100µg/ml of API. Using a Shimadzu UV-1800 spectrophotometer, we scanned the carvedilol, furosemide, and nimodipine stock solutions throughout a 200–400 nm wavelength range after further diluting them with solvent to produce various concentrated solutions.21, 22, 23
Solubility study
We performed the solubility investigation using the methodology outlined by Higuchi and Connors (1965). Dendrimers of full generation TG1.0, TG2.0, and TG3.0 were introduced to screw-capped vials containing excess API (carvedilol, furosemide, and nimodipine) at quantities ranging from 0.6 mmole to 3 mmole. The vials were subjected to a 37ºC shaking water bath for a duration of 48 hours. A Shimadzu UV-1800 spectrophotometer was used to measure the absorbance of carvedilol, furosemide, and nimodipine at their characteristic wavelengths of 284.50 nm, 341.50 nm, and 356.50 nm, respectively. The vials were centrifuged to eliminate any undissolved API.24
Hemolysis assay
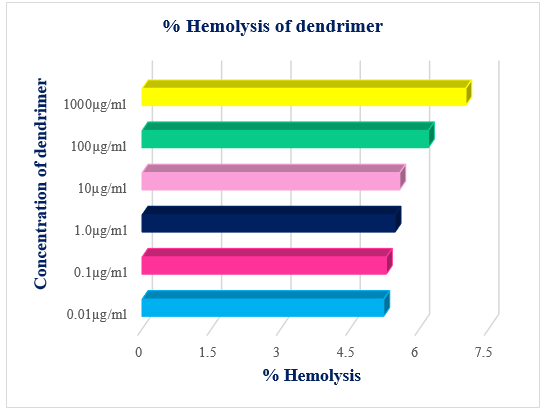
An investigation was conducted to determine the hemolytic effect of increasing Nanostructure dendrimer concentrations on RBC suspension. Blood samples were briefly spun in a centrifuge at 1500 rpm for 5 minutes. The blood cells were resuspended in 0.5 ml of PBS and treated at 37ºC for 1 hour with different dendrimer concentrations (0.01, 0.1, 1.0, 10, 100, and 1000 µg/ml) after centrifugation, with the supernatant plasma discarded. Following incubation, the samples were spun in a centrifuge to collect the supernatant. This was then mixed with a volume of Phosphate buffer saline (pH 7.4) to dilute the samples. Absorbance was measured at 540 nm against the normal saline supernatant. Each sample's percent haemolysis was calculated by treating the water absorbance as a 100% haemolytic sample. The hemolytic impact of different dendrimer concentrations was determined using the formula.25
% Hemolysis= (Absorbance of test/Absorbance of control)*100
Determination of Cell Viability: MTT Assay
Human A549 non-small cell lung cancer cells were grown at 37°C in a CO2 incubator (5% CO2) in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% Fetal Bovine Serum (FBS) and 1% Antibiotic-Antimycotic solution. Cell viability was determined by collecting cultivated cells with trypsin and seeding them in a 96-well plate at a density of 10,000 cells per well and incubating for a period of twenty-four hours. Following incubation, cells were treated for 24 hours with various concentrations (10, 100 and 1000, µg/ml) of dendritic macromolecules. The control wells received DMEM. After 24 hours, medium with various dendrimer concentrations was removed from each well, and cells were treated with 0.5mg/ml MTT for 4 hours at 37ºC. Following incubation, MTT was withdrawn and DMSO (100µl/well) was applied to dissolve the formazan crystals. The absorbance of each well was measured at 550nm with an ELISA plate reader, and the % cell viability was determined using the following formula.25
% Cell Viability= (Mean Absorbance of test/Mean Absorbance of Vehicle control)*100.
Result & Discussion
Preparation mechanism
Prior research has documented the synthesis and characterisation of dendrimers TG1(OH)8, TG2(OH)32, and TG3(OH)128 derived from thiocarbamide. Water solubility was observed in full generation dendrimers TG1(OH)8, TG2(OH)32, and TG3(OH)128 but not in half generation dendrimers or core compound. Research using A-549 lung cancer cell lines showed that TG3.0 dendritic macromolecules synthesized in-house outperformed PAMAM dendrimer, a commercially available product, in hemolysis and cytotoxicity tests. As a result, medication solubilization only used whole generation dendrimers.20
Λmax of drugs
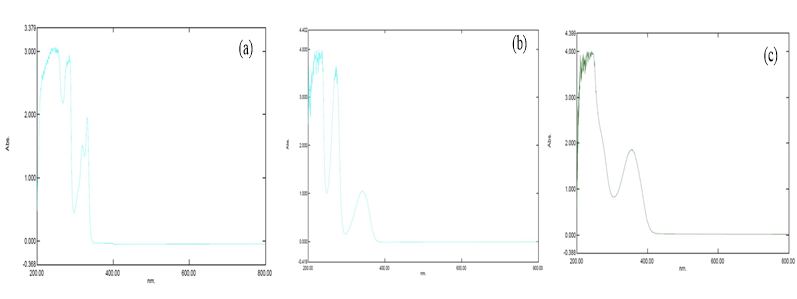
The carvedilol, furosemide and nimodipine content was analysed using UV-visible spectroscopy and determine the λmax at 284.50 nm, 341.50nm and 356.50nm respectively and correlation coefficient r2 value in calibration curve is 0.99188, 0.99875 and 0.99808 for carvedilol, furosemide and nimodipine respectively. λmax of carvedilol, furosemide and nimodipine are shown in the Figure 1 a-c respectively.
Drug solubility study
Poor water solubility of antihypertensive API (carvedilol, furosemide and nimodipine) is a major issue in the successful development of effective dosage forms. Solubility measurements were performed according to the method of Higuchi and Connors. 24 In the present investigation, the aqueous solubility of carvedilol was found to be 9.458 µg/ml. The influence of the dendrimer generation on the apparent solubility of carvedilol in water was investigated by phase solubility diagrams. At 37°C temperature, the effect of triazine based nanostructured dendrimer concentration on solubility of carvedilol was studied, and the findings are presented in figure 2a. According to our results solubility of carvedilol has been significantly improved by dendritic macromolecules. The solubility of carvedilol increased linearly with increasing dendrimer concentration over the concentration range 0.6mM to 3.0mM. It was discovered that carvedilol’s solubility improved up to the 147.065µg/ml by 3.0mM TG3.0 dendrimer. From the Figure 2 b it was clear that the solubility of carvedilol was affected by the different generation of dendritic macromolecules. carvedilol's solubility improved by 3.0mM TG3.0 dendrimer at pH 1.2 is 154.339µg/ml. FTIR analysis was performed to analyse the physiochemical interaction between the different ingredient of the formulation and the drug. The presence of drug was confirmed by FT-IR on TG3.0 dendrimer to identify the drug containing dendrimer. we compared the FT-IR spectra of pure TG3.0 dendrimers and drug to those of drug-containing dendrimers. FT-IR spectrum of pure TG3 dendrimer showed absorption bands 3314 cm-1 for O-H stretching for hydroxyl groups, 1054 cm-1 for C–O stretching, 1456 cm-1 for C=S stretching, 1652 cm-1 for C=N stretching. FT-IR spectrum of pure carvedilol showed absorption bands at 3345.04 cm-1 for O-H and N-H stretching vibration peaks, 2923.48 cm-1 for C-H stretching vibrations, 1591.03 cm-1 for N-H bending vibrations and 1252.66 cm-1 for O-H bending and C-O stretching vibrations. The presence of almost all characteristics peaks of drug in drug containing dendrimer showed absorption bands at 3307.79 cm-1 for O-H and N-H stretching vibration peaks, 2929.08 cm-1 for C-H stretching vibrations, 1555.16 cm-1 for N-H bending vibrations and 1242.34 cm-1 for O-H bending and C-O stretching vibrations. There was no major deviation in peak of the FTIR spectrum for the optimized drug containing dendrimer and pure carvedilol.
Figure 2
(a) Solubility enhancement of carvedilol by TG3.0 (b) Effect of dendrimer generation on carvedilol solubility
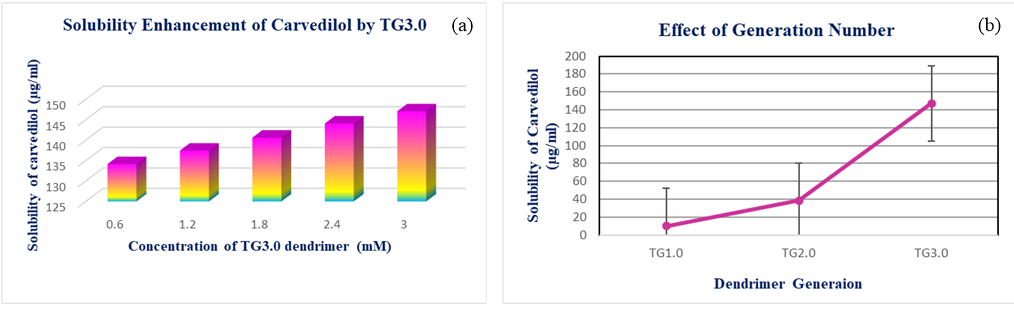
The aqueous solubility of furosemide was found to be 6.184 µg/ml. The influence of the dendrimer generation on the apparent solubility of furosemide in water was investigated by phase solubility diagrams. At 37°C temperature, the effect of triazine based dendrimer concentration on solubility of furosemide was studied, and the findings are presented in Figure 3 a. According to our results solubility of furosemide has been significantly improved by dendritic macromolecules. The solubility of furosemide increased linearly with increasing dendrimer concentration over the concentration range 0.6mM to 3.0mM. It was discovered that furosemide’s solubility improved up to the 70.999µg/ml by 3.0mM TG3.0 dendrimer. From the Figure 3 b it was clear that the solubility of furosemide was affected by the different generation of dendritic macromolecules. furosemide’s solubility improved by 3.0mM TG3.0 dendrimer at pH 7.4 is 77.769µg/ml. FT-IR spectrum of pure furosemide showed absorption bands for N-H stretching at 3400 cm-1, NH stretching in sulphonamide at 3351.20 and 3284.90 cm-1. The C=O stretching, the vibration was at 1673.18 cm-1, S=O stretching, vibration at 1142.70 cm-1 and 1323.91 cm-1, most important stretching vibration was OH stretching at 3122.70 cm-1, NH bending at 1564.60-1592.25 cm-1. The presence of almost all characteristics peaks of drug in drug containing dendrimer, NH stretching in sulphonamide at 3360 cm-1,C=O stretching, the vibration was at 1668.42 cm-1, S=O stretching, vibration at 1122.66 cm-1 and 1361.82 cm-1, most important stretching vibration was OH stretching at 3122.97 cm-1, NH bending at 1554cm-1. There was no major deviation in peak of the FTIR spectrum for the optimized drug containing dendrimer and pure furosemide.
Figure 3
(a) Solubility enhancement of furosemide by TG3.0 (b) Effect of dendrimer generation on furosemide solubility.
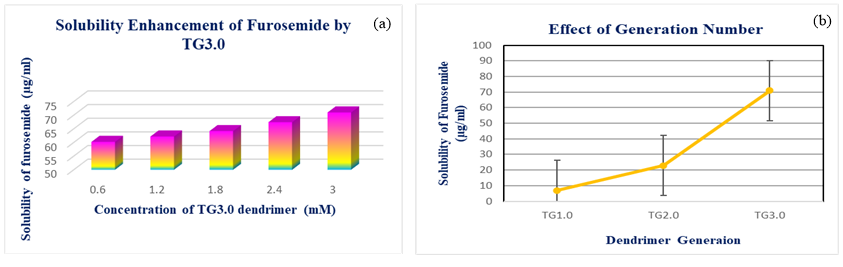
The aqueous solubility of nimodipine was found to be 2.198 µg/ml. The influence of the dendrimer generation on the apparent solubility of nimodipine in water was investigated by phase solubility diagrams. At 37°C temperature, the effect of triazine based dendrimer concentration on solubility of nimodipine was studied, and the findings are presented in Figure 4 a. According to our results solubility of nimodipine has been significantly improved by dendritic macromolecules. The solubility of nimodipine increased linearly with increasing dendrimer concentration over the concentration range 0.6mM to 3.0mM. It was discovered that nimodipine’s solubility improved up to the 38.08µg/ml by 3.0mM TG3.0 dendrimer. From the Figure 4 b it was clear that the solubility of nimodipine was affected by the different generation. FT-IR spectrum of pure nimodipine showed NH stretching of aliphatic secondary amine at 3272.40cm-1, H bending of aliphatic secondary amine at 1644.23cm-1, C-H stretching of benzene ring at 3088.33cm-1, C-H asymmetric stretching of methyl at 2978.42 cm-1, C-H asymmetric stretching of methylene at 2950.07 cm-1 carbonyl stretching of ester at 1697.67cm-1, C=C stretching of benzene ring at 1624.71 cm-1,1529.92cm-1 and 1498.67 cm-1, NO2 asymmetric stretching at 1454.74cm-1, NO2 symmetric stretching at 1306.11 cm-1. The presence of almost all characteristics peaks of drug in drug containing dendrimer showed NH stretching of aliphatic secondary amine at 3360.58cm-1, H bending of aliphatic secondary amine at 1667.82cm-1, C-H asymmetric stretching of methyl at 2927.85cm-1, C=C stretching of benzene ring at 1613.91 cm-1, 1555.50cm-1 and 1462.08cm-1, NO2 asymmetric stretching at 1454.40cm-1, NO2 symmetric stretching at 1360.90cm-1. There was no major deviation in peak of the FTIR spectrum for the optimized drug containing dendrimer and pure nimodipine.
Figure 4
(a) Solubility enhancement of nimodipine by TG3.0 (b) Effect of dendrimer generation on nimodipine solubility
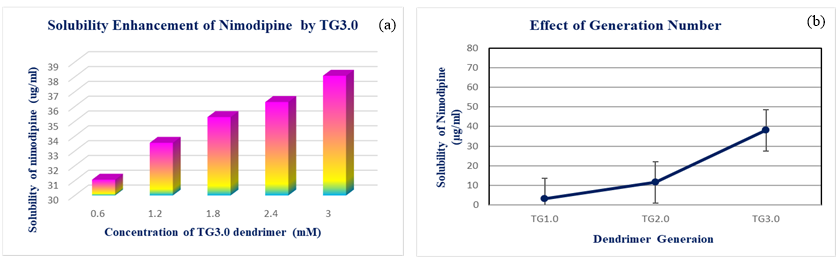
The solubility improvement effect of various dendritic generation used in this study followed the order: TG3.0 > TG2.0 > TG1.0. solubility stability of drug containing dendrimer checked for 3 weeks and there is no major difference in the absorbance. increase drug solubility most likely due to a cavity, hydrogen bonding, and electrostatic contact between dendrimer terminal functional groups and drug molecules. This research revealed that dendrimer showed better result than ꞵ-CD in increasing the solubility of carvedilol, furosemide and nimodipine. 26, 27, 28 Overall characteristic bands for both TG3.0 dendrimer and API remained unchanged in IR spectrum of API loaded dendrimer. As a result, dendrimer comprises a hydrophobic triazine ring in the interior that may impart hydrophobic interaction and hydroxyl groups on the outside that can promote hydrogen bonding. As a result, dendrimers may have increased drug’s solubility and encapsulation via hydrophilic interactions, hydrogen bonding, or both.
Hemolytic potential
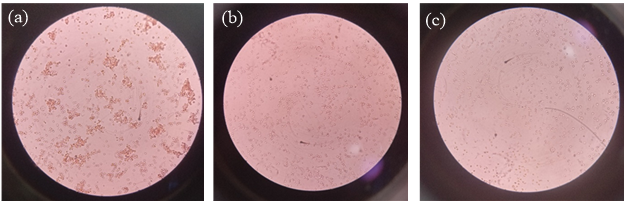
Effects of hydroxyl-terminated dendritic macromolecules on the erythrocytes are shown in Figure 5. The results indicated that hydroxyl-terminated dendritic macromolecules caused hemolysis in a concentration-dependent manner. However, triazine based TG3.0 dendrimer were significantly better hemolysis result compared to PAMAM dendrimer.29 Hemolysis is caused by an interaction between the positively charged amine groups of the PAMAM dendrimer and the negatively charged surfaces of erythrocytes (RBCs).30 In comparison, TG3.0 dendrimers have anionic hydroxyl groups on the surface, which reduces interaction with erythrocytes (RBCs) and results in significantly lower toxicity. Microscopic image of hemolysis study is shown in Figure 6.
Cytotoxicity result
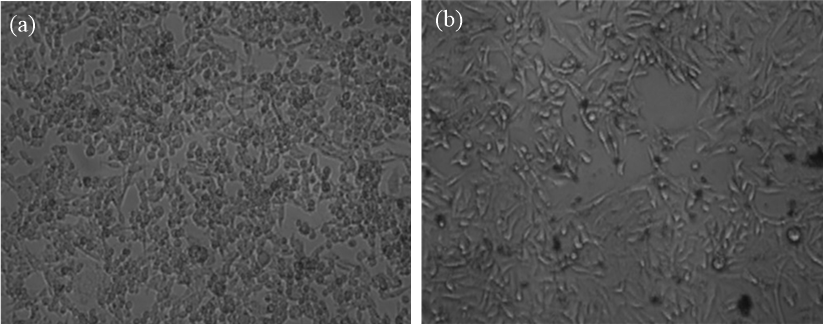
The MTT assay technique was used to investigate the cellular toxicity of TG3.0 dendritic macromolecules on A-549 cell lines. MTT is a yellow dye that is easily soluble in water. MTT can be transformed into water-insoluble, blue-colored formazan crystals by reductive breakage of the tetrazolium ring by living cells. Formazan crystals recovered with organic solvents and evaluated at a wavelength of 550 nm, and result are correlated with living cells to determine cell viability. Cytotoxicity revealed that TG3.0 dendrimer had greater than 90% cell viability at concentrations ranging from 10 µg/ml to 1000 µg/ml. As a result, TG3.0 dendrimer was far less cytotoxic. The morphology of A-549 cell lines when treated with control and varied dendrimer concentrations is shown, exhibiting a reduction in cell density with increasing dendrimer concentration from 10 µg/ml to 1000 µg/ml.Figure 7 are shown microscopic image of control and 1000 µg/ml dendrimer concentration respectively.
Conclusion
To improve the oral bioavailability of nimodipine, furosemide and carvedilol several generations of dendritic macromolecules were produced. Dendritic macromolecules were shown to be much less toxic and biocompatible in the cytotoxicity and hemolytic experiments, suggesting that they might be used as a potential drug carrier system. The production of dendritic macromolecules and concentration determine how well a medication dissolves in aqueous solution. The current investigation shows that TG3.0 dendrimer has a significant potential to improve carvedilol, furosemide, and nimodipine solubility. When it came to improving the solubility of carvedilol, furosemide, and nimodipine, dendrimer outperformed ꞵ-CD.
Declaration of Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work would not have been possible without the support of the following institutions and individuals: Nirma University's Department of Pharmacy, the Principal of the Institute of Science and Technology for Advanced Studies and Research (ISTAR), and V.P. & R.P.T.P. Science College. The authors are grateful to Aim Chemicals, Vapi, Gujarat, for supplying the API. The spectroscopic analysis capability was provided by Sophisticated Instrumentation Centre for Advanced Research and Testing (SICART), CVM University, Vallabh Vidyanagar and Centre of excellence (A division of Vapi green enviro limited), Vapi, Gujarat.