Introduction
Melanoma is recognized as a highly perilous type of skin tumor, characterized by rapid metastasis, progression, and a significant mortality rate, particularly when diagnosed at an advanced stage. Melanoma and non-melanoma (basal cell carcinoma and squamous cell carcinoma) are the two subtypes of this condition, with melanoma being more dangerous than the latter. Melanocytes are found across several regions of the body, including the skin, eyes, nasal passages, oral and pharyngeal mucosa, as well as the vaginal and anal mucosa.1
The Extracellular signal-regulated kinase (ERK) pathway, which is also called the Ras/Raf/Mitogen-activated protein kinase/ERK kinase (MEK)/extracellular-signal-regulated kinase (Ras/Raf/MEK/ERK) signal transduction system, is often turned on in cancer because of a number of abnormal molecular changes that are not under control. This signalling pathway regulates various essential cellular activities such as cell viability, reproduction, movement, and specialization. It is consistently active in lung, colon, pancreatic, kidney, and ovarian malignancies. Moreover, the ERK pathway contains some of the most well-defined oncogenic alterations, such as Ras and B-Raf. ERK plays a crucial role in the signalling pathway that occurs after Ras, Raf, and MEK. It acts as a central hub where several signalling pathways come together to stimulate transcription.2
Database of naturally occurring plant-based compounds with anti-cancer properties and their corresponding activities and targets. The Naturally Occurring Plant-based Anti-cancer Compound-Activity-Target (NPACT) database is a meticulously selected collection of natural chemicals produced from plants that demonstrate anti-cancer properties. The dataset has 1574 entries, and each entry has detailed information about the structure, composition (physical, elemental, and topological), type of cancer, cell lines, inhibitory values Half maximal inhibitory concentration, effective concentration, median effective dose (IC50, EC50, ED50), molecular targets, commercial suppliers, and how similar the compounds are to drugs. NPACT focuses exclusively on the study of natural chemicals derived from plants that have anti-cancer properties. NPACT stands out by offering the bioactivities of these natural compounds against various cancer cell lines and their corresponding molecular targets.3
The present work aims to investigate the anticancer properties of NPACT ligands against the ERK signalling pathway in melanoma. The hit compounds acquired in this investigation could have a significant impact on the development of novel medications that specifically target this cancer.
Materials and Methods
General
The present investigation utilized the following software: i) Autodock 4.2.6, ii) MGLTools 1.5.6, iii) Babel-2.4.0, iv) BIOVIA Discovery Studio 4.5, v) Avogadro Molecular Mechanics Force Fields (MMFF94), vi) Chemdraw Ultra 8.0, vii) Java Platform, and viii) SwissADME online tool. The SwissADME online tool was utilized to evaluate the pharmacokinetic characteristics of the ligands (http://www.swissadme.ch).
Ligand preparation
The ligands utilized in this work were obtained from the NPACT database (http://crdd.osdd.net/raghava/npact/) in SDF format. Subsequently, Babel-2.4.0 software was employed to convert the ligands to pdb format. The Avogadro software was then utilized to do energy minimization of the ligands. The ligands were modified by introducing hydrogen bonds and assigning rotatable bonds and Gasteiger charges using Autodock 4.2.6 software. The modified ligands were then saved in (Protein Data Bank, Partial Charge (Q), & Atom Type (T)) PDBQT format for subsequent docking procedures.
Protein preparation
Structure of the ERK protein (Figure 1) was retrieved from protein data bank PDB (PDB ID: 2OJG).4 The structure of 2OJG in complex with an inhibitor was prepared using Autodock 4.2.6 software, by adding polar hydrogens, merging non-polar hydrogens, assigning Kollmann charges, and saving the file in PDBQT format.
Molecular docking
Autodock Vina program was performed between the ligands and protein target for molecular docking analysis such as binding type, binding energy, inhibition activity, ligand efficiency, distance, and possible interactions. Their molecular docking scores were set as Autodock 4.2.6 tools and BIOVIA Discovery Studio software of the Molecular Graphics Laboratory software package by keeping the analogue flexible. 5, 6 The ligand efficiencies, binding energies, inhibition activity, hydrogen bonds, and bond lengths of the protein-ligand complexes were analysed using Autodock 4.2.6 software. BIOVIA Discovery Studio software was used for visual inspection of docking poses.7
Absorption, Distribution, Metabolism, Elimination & Toxicity (ADMET) Prediction
The pharmacokinetic and toxicity properties of the 9 compounds were achieved by using the SwissADME, which is an open online tool (http://www.swiss adme.ch). The ADME properties define blood-brain barrier (BBB) permeability and passive human gastrointestinal absorption (HIA) as well as a substrate or non-substrate permeability glycoprotein (P-gp) and Cytochrome P450 (CYP).
Results
Table 1
Molecular docking score andhydrogen bond interactions of 2O JG and the ligand complexes
Table 2
Drug-likeness properties of the ligands
Figure 1
3D & 2D interactions of piceatannol with the target protein (2OJG), a ERK receptor in melanoma
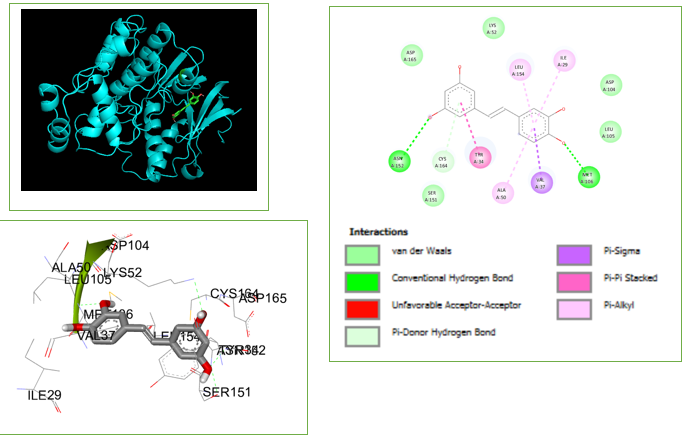
Piceatannol, also known as trans-2,3′,4′, 5-tetrahydroxystilbene, is a phenolic molecule belonging to the stilbenoid class. It is a hydroxylated derivative of resveratrol. Piceatannol is derived from important sources such as grapes, passion fruit, white tea, Japanese knotweed, Asian legumes, and Korean rhubarb. Due to its lower concentration in grapes and wines compared to resveratrol, piceatannol has garnered significantly less scientific focus.8
Studying natural products, such as herbs and medicinal plants, is crucial due to their therapeutic potential and the important pharmacological compounds they contain.
These molecules have been optimized through the process of evolution to mimic pharmaceuticals and remain the main source of medications and potential therapeutic candidates. Out of a pool of 1574 compounds, we have chosen 464 natural compounds by applying Lipinski's rule of five. An assessment of the compound's druggability was conducted by utilizing online tools (Swiss ADME) to analyze its structure. An analysis was conducted on the features pertaining to the compound's suitability as a medication, and the findings are displayed in Table 1. The Autodock software was utilized to conduct molecular docking. The docking investigation revealed the presence of (PDB code: 2OJG). It's clear that both compounds have a strong interaction with MET 106 and ASN 152, as they form two hydrogen bond interactions (Table 2). Figure 1 illustrate the maximum binding affinities that can occur between piceatannol and the 2OJG receptor binding sites. The molecular docking study showed that the chemical piceatannol had a stronger attraction to the ERK receptor's binding site. Based on the binding energy estimates, the new drug has a strong affinity for the binding pocket, which makes it very effective against the ERK receptor in melanoma.
Conclusion
Piceatannol has demonstrated favourable pharmacological characteristics, making it a promising candidate for the treatment of melanomas. This was identified using drug-likeness and ADMET predictions. The compound was determined to be non-toxic and had favourable interactions with the target protein. Consequently, its potential as an anticancer agent for the treatment or suppression of melanoma warrants further investigation in future experimental research.
