Introduction
Psoriasis is a multifaceted condition affecting approximately 2% of the global population, with higher prevalence observed in Caucasian, Asian, and Black demographics. It exhibits geographical variation, being more common in colder northern regions and less prevalent in tropical climates in Europe.1 The disease can manifest at any age and is characterized by intricate pathophysiological mechanisms. Psoriasis, an enduring autoimmune skin disorder, has been recognized since ancient times and is typified by the gradual enlargement and shedding of psoriatic plaques alongside pustules and spots, originating in the papillary dermis. Anomalies in keratinocyte proliferation and migration result in epidermal thickening, incomplete differentiation, and parakeratosis. 2 As the condition progresses, certain skin cell populations excessively proliferate, leading to acanthosis nigricans. Typically, psoriasis presents as red, inflamed patches with silvery-white scales, often symmetrically distributed. It is classified as an autoimmune ailment and is associated with immune system dysfunctions involving T cells, dendritic cells, and cytokines like interleukin 23, interleukin-17, and tumor necrosis factor. Psoriasis stands as the most common genetic skin disorder and correlates with heightened risks of metabolic syndrome and cardiovascular disease. Severe forms of psoriasis are linked with components of metabolic syndrome, including obesity, dyslipidemia, diabetes mellitus, and hypertension. 3 Despite conventional therapies providing symptomatic relief, a definitive cure remains elusive, with many treatments carrying adverse effects such as atrophy, organ toxicity, immunosuppression, infection, and carcinogenesis.4
Recent years have witnessed growing interest in drug carriers, offering solutions to various diseases by addressing issues such as low bioavailability, poor solubility in water, and biological degradation of medicinal substances. Topical drug delivery systems are frequently employed for localized effects on the skin, catering to cosmetic and dermatological needs, including the treatment of skin disorders like eczema, acne, and psoriasis. 5 These systems, however, can also exert systemic effects through transdermal delivery, enhancing patient compliance and facilitating self-medication. Hydrogels emerge as modern and versatile drug delivery vehicles owing to their highly adjustable physical properties. 6 Comprising primarily water and a polymer forming a three-dimensional network structure through chain cross-linking, hydrogels exhibit porosity, accommodating drug molecules. Nonetheless, a notable drawback of hydrogels is their inability to deliver hydrophobic drugs, a major challenge given the hydrophobic nature of many effective drug substances. To overcome this limitation, emulgels, also known as creamed gels and gelled emulsions, have been developed. Emulgels represent innovative drug delivery systems formed by blending emulsions and gels, possessing characteristics of both while offering dual-controlled release capabilities, alongside numerous desirable attributes and high patient acceptability. 7
In recent years, emulgels have emerged as promising delivery platforms for both hydrophilic and lipophilic functional compounds and nutraceuticals, including carotenoids, vitamins, probiotics, and unsaturated fatty acids. These systems offer solutions to challenges such as low chemical stability, limited solubility, and poor absorption of food ingredients, while also enhancing the sensory qualities of formulations, bioavailability, bio-accessibility, and controlling their release. 8, 9 Researchers have shown particular interest in protein-based emulsions derived from sources like casein, soy, gelatin, and whey protein due to their effective emulsifying and gelling properties, as well as their abundance and renewable potential. Emulgels designed for food applications offer advantages over traditional emulsions, including slower release of active compounds in the intestines due to improved stability in gastric and intestinal environments. This enhanced stability is attributed to structural changes in emulsion gels during digestion, which inhibit the action of lipases on oil droplet surfaces, thereby reducing lipid digestion and the release of incorporated ingredients. 10 Through careful selection of emulgel excipients, desired release profiles can be achieved in a controlled manner. Moreover, ongoing research explores the development of emulgels as stimuli-responsive systems, capable of altering their morphology and properties in response to external stimuli. 11
The article presents scientific findings supporting the efficacy of natural substances in the treatment of Psoriasis. It comprehensively examines in vitro, in vivo, and clinical studies investigating plant-derived compounds and their effects on psoriatic symptoms, immunomodulation, and anti-inflammatory responses. Providing detailed insights into psoriasis pathogenesis, inflammation, oxidative stress, and molecular interactions between natural compounds and the immune system, the article aims to enhance understanding of their therapeutic potential. Moreover, the review explores the utilization of natural products and emulgels as potential treatments for psoriasis. It delves into their effectiveness, mechanisms of action, and clinical applications in managing psoriasis, focusing on their therapeutic benefits and potential impact on the treatment landscape for this skin condition. By synthesizing evidence from various studies, the article seeks to shed light on the role of natural substances and emulgels in psoriasis management, offering valuable insights for researchers and clinicians alike.
Pathogenesis of Psoriasis
Psoriasis's origins are intricate, stemming from an abnormal immune response in the skin influenced by both genetic factors and environmental triggers. Treatment primarily focuses on addressing autoimmune-related mechanisms involving immune system cells and cytokines. The skin's microbiota contributes to immune system regulation, while genetic predispositions are significant contributors to psoriasis inheritance. Mutations in regulatory proteins within immune cells result in altered cytokine expression, impacting the proliferation and differentiation of keratinocytes. Variants of immune cells and proteins linked to psoriasis contribute to varying disease courses. Epigenetic modifications, including DNA methylation, histone modifications, and non-coding RNAs, represent a recent addition to our understanding of psoriasis pathophysiology. 12, 13, 14
Immune system dysfunction and psoriasis
A defining trait of psoriasis is persistent inflammation, which triggers the excessive proliferation and irregular differentiation of keratinocytes. Histologically, psoriatic plaques exhibit epidermal hyperplasia, accompanied by inflammation comprising dermal dendritic cells, macrophages, T cells, and neutrophils. In psoriatic alterations, two distinct sets of cellular responses disrupt the equilibrium between innate and adaptive immune cell activation and the factors secreted by keratinocytes, consequently impacting T cells and dendritic cells directly.
Key players in congenital immunity during psoriatic changes include neutrophils, plasmacytoid dendritic cells (pDCs), and CD11c+ dendritic cells. Neutrophils undergo continuous production in the bone marrow and subsequent transportation to the bloodstream. Chemokines such as interleukin-8 (IL-8), CXCL1, and proteins like S100A7/A8/A9, released by keratinocytes, establish a chemotactic gradient facilitating neutrophil migration to the epidermis. PDCs, characterized by BDCA-2+ and CD123+ antigen expression, are known for their high production of interferon-α (IFN-α) upon activation, and are thought to play a pivotal role in initiating disease-related changes.
The presence of abundant T cells and mature dendritic cells (DCs) in skin aggregates, along with the expression of lymphoid-attracting chemokines such as CCL19, CCL21, CXCL12, and CCL18, may facilitate the activation of T cells within the skin itself. T cells in psoriatic alterations are classified into two functional groups: helper T cells (TH) and cytotoxic T cells (TC). A subset of T cells expresses CD161 and other cytotoxic receptors, indicating the involvement of natural killer T cells in psoriasis pathogenesis. Cytokine interactions in psoriasis are thought to follow a model where inducers such as IL-23 or IL-12 trigger the production of IFN-γ and TNF by T cells, leading to the activation of numerous IFN-responsive genes via signal relay and the transcription activator STAT1. However, this model represents only a fraction of the over 1300 genes positively regulated in psoriatic changes. Activated DCs may collaborate with IFN-α, IL-20, IL-12, and IL-23 in an inflammatory cascade. Additionally, other cytokines produced by keratinocytes or stromal cells likely regulate the proliferation and fibrosis of epithelial-stromal (vascular) structures in psoriatic lesions. 15, 16
Oxidative stress and psoriasis
Oxidative stress is imbalance between reactive oxygen species and antioxidant system. 17 Numerous studies have demonstrated the involvement of reactive oxygen species (ROS) and nitric oxide synthases (NOS) in the pathogenesis of psoriasis. The imbalance in redox status and elevated levels of inducible NOS contribute to the generation of oxidative stress, which can result in various molecular abnormalities. ROS encompass a diverse group of oxygen-containing molecules capable of reacting with other substances, and they can be categorized into radicals, including hydrogen peroxide (HO2•), superoxide (O2• −), hydroxyl (OH•), and peroxyl radicals (RO2•), as well as non-radicals such as ozone (O3), hydrogen peroxide (H2O2), and hypochlorous acid (HOCl)(18).
Nitric oxide synthases (NOS) are enzymes responsible for catalyzing the synthesis of nitric oxide (NO), with three isoforms: endothelial NOS (eNOS), neuronal NOS (nNOS), and inducible NOS (iNOS). In psoriasis, iNOS is excessively synthesized by various cell types, including keratinocytes, compared to healthy skin cells, implicating this isoform in the disease's pathogenesis. Oxidative stress arises when the balance between reactive oxygen species (ROS) and antioxidants tilts in favor of ROS, disrupting redox signaling and regulation, and leading to various molecular abnormalities. Studies demonstrate that oxidative stress induced by varying levels of ROS can result in DNA modifications, lipid peroxidation, and the release of proinflammatory cytokines. As second messengers, ROS influence cellular signaling pathways, particularly proinflammatory signaling pathways, and the expression of numerous genes. The human body possesses mechanisms to counteract oxidative stress factors even before they exert detrimental effects on cells. Skin enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) play critical roles in the antioxidant defense process. Non-enzymatic antioxidants like vitamins E and C, as well as glutathione (GSH), also contribute significantly to antioxidant defense.
In the pathogenesis of psoriasis, mild oxidative stress holds greater significance than severe oxidative stress. There exists a positive correlation between oxidative stress markers and the psoriasis area and severity index (PASI), while an inverse relationship is observed between antioxidant markers and PASI scores. Factors such as total oxidative stress (TOS), plasma or serum malondialdehyde (MDA) levels, and the release of vascular endothelial growth factor (VEGF) from various cell types play pivotal roles in angiogenesis in psoriasis. Additionally, VEGF may drive leukocyte migration through psoriatic skin, thereby exacerbating the inflammatory process. 18, 19, 20
Rationale for Exploring Natural Products and Emulgel in Psoriasis Therapy
Emulsion gels have emerged as essential pharmaceutical topical semi-solid dosage forms since the 1980s. These gels, categorized as either water-in-oil (W/O) or oil-in-water (O/W) emulsions, are solidified with the assistance of one or multiple gelling agents, constituting two main components: the emulsion and the gel. The incorporation of a gelling agent into the aqueous phase transforms a conventional emulsion into an emulgel, rendering it thixotropic and enhancing the system's bioavailability. 21
The emulsion component functions as a controlled release system, where drug particles are entrapped within the internal phase. The dispersed phases within the emulsion serve as reservoirs of the drug, gradually releasing it in a controlled manner through the external phase onto the skin. 22 Meanwhile, the gel component forms a cross-linked network, trapping small drug particles and facilitating their controlled release. Additionally, the mucoadhesive property of the gel extends the contact period of the medication on the skin, further enhancing its effectiveness. While gels offer rapid drug release compared to other semisolid preparations, they face limitations in delivering hydrophobic drugs. 23 To address this challenge, emulgels are developed, allowing even hydrophobic drugs to benefit from the advantageous properties of gels. By incorporating an emulsion into a gel matrix, emulgels function as dual-controlled release systems, mitigating issues like phase separation and creaming commonly associated with emulsions.
Figure 1
Diagrammatic representation showing applicability of emulgel formulation for phototherapy of Psoriasis
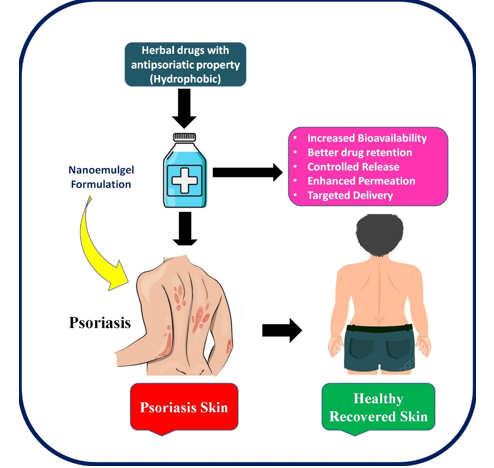
Emulgels are simple to prepare and cost-effective to manufacture. Their preparation involves simple steps without requiring specialized equipment, and the materials used are readily available and inexpensive. 24 Emulgels can be formulated in two ways: by blending a gelling agent with an aqueous phase or by introducing a gelling agent as a component of the water phase in the emulsion. This versatility in preparation methods contributes to the widespread adoption and practicality of emulgels in pharmaceutical formulations. 25 Emulgels combine the advantages of both emulsions and gels, making them highly favored by patients. They offer numerous benefits as transdermal formulations, including controlled drug release and convenient administration, which improve patient adherence to treatment. 26 Furthermore, the rheological and sensory properties of emulgels enhance their application to the skin. Emulgels designed for dermatological purposes exhibit several desirable properties. They are thickotropic, non-greasy, easily spreadable, and readily removable. Additionally, they provide emollient effects, do not stain clothing, are water-soluble, biocompatible, transparent, have extended shelf lives, and boast pleasing aesthetics. These systems share similarities with the lipoprotein structure of the skin, enabling them to spread more easily over large areas, such as psoriatic lesions, compared to creams or ointments. 27
In summary, emulgels have proven effective in treating psoriasis, thanks to their stability, user-friendly application, and compatibility with diverse drug delivery methods. The selection of an ideal consistency of gelling agents is paramount, as it directly impacts the mechanical and sensory characteristics of the formulation, as well as the comfort of use for patients. By carefully considering these factors, emulgels can continue to serve as valuable tools in managing psoriasis effectively. The Diagrammatic representation of applicability of emulgel formulation for phototherapy of Psoriasis shown in.Figure 1
Natural Products in Psoriasis Treatment
a) Aloe vera
Aloe vera, a well-known plant with applications in burn treatment and skincare, contains six antiseptic compounds—lupeol, salicylic acid, urea nitrogen, cinnamonic acid, phenols, and sulfur—that individually inhibit the growth of fungus, bacteria, and viruses.28 Psoriatic plaques were successfully removed in 83.3% of treated patients in a 1995 double-blind, placebo-controlled research involving 60 patients with psoriasis vulgaris when aloe vera extract (0.5%) was added to a hydrophilic cream, as opposed to 6.6% in the placebo group. Aloe vera, a perennial succulent belonging to the Liliaceae family, produces a colorless, mucilaginous gel with various potential pharmacological actions, including anti-inflammatory properties. This gel, used for cosmetic and therapeutic purposes, has been deemed a successful psoriasis treatment without any drug-related adverse effects. 29, 30
Curcumin
Curcumin, a constituent found in turmeric with a rich history of use in Southeast Asia, has been recognized for centuries for its diverse medicinal properties, including antioxidant, anti-inflammatory, antimicrobial, and anticancer effects. 31, 32 Extensive research has established curcumin as a safe and non-toxic substance for humans, with doses of up to 8 g per day considered well-tolerated. 33 Its potential efficacy in combating psoriasis has been explored, with molecular docking studies revealing its interaction with TNF-α receptor-binding sites. This interaction, involving residues like Leu89, Asn90, Asp105, Asn106, and Cys129, indicates that curcumin directly influences TNF-α, disrupting signal transduction and suppressing inflammation induced by this cytokine. 34
Clinical trials have demonstrated the tolerability of oral curcumin in psoriasis patients, although conclusive evidence requires extensive, placebo-controlled trials. While overall response rates were modest, promising results in specific individuals suggest further investigation. 35 In vivo studies on mice by Tu et al.indicated that curcumin inhibits TLR2, TLR4, and TLR-9 expression, potentially reducing proinflammatory cytokine levels and enhancing the anti-inflammatory IL-10. This implies that curcumin may be used to treat inflammatory illnesses by acting as an immunomodulatory and anti-inflammatory drug. 36 Curcumin suppresses TNF-induced production of IL-1ß, IL-6, and TNF in TNF-treated HaCaT cells, according to in vitro studies by Cho et al. Curcumin's potential as an immunomodulatory drug is highlighted by the correlation between its inhibitory impact on cytokine production and the reduction of MAPKs and NF-B. Curcumin has been shown by Sun et al. to suppress TNF-induced NF-B activation and the production of IL-6/8 in HaCaT cells, suggesting its role in minimizing keratinocyte-related inflammation through NF-B inhibition. 37
Additionally, curcumin has demonstrated potential as a phosphorylase kinase activity (PhK) inhibitor. PhK activity is linked to psoriatic activity. Curcumin has the ability to cure psoriasis locally, as evidenced by the reduced PhK levels seen in platelet samples treated with a water-alcohol extract from the rhizome of turmeric.. 38 Curcumin has been shown in vitro to suppress proinflammatory cytokines and keratinocyte proliferation in a variety of cell types, indicating that it may find use in the treatment of hyperproliferative disorders such as psoriasis. Curcumin was discovered to suppress TNF's anti-apoptotic action, hence preventing the progression of psoriasis in HaCaT cells treated with TNF-α. 39
Genistein
Genistein, a flavonoid commonly found in various vegetables such as soybeans and fava beans, occurs in food at concentrations ranging from 1 to 2 mg/g. Extensive research has elucidated its manifold biological effects, encompassing antioxidant, antiangiogenic, and anticancer properties. 40 In vivo investigations by Wang et al. demonstrated that in a mouse model of psoriasis, administration of genistein at doses of 50 and 100 µM for 2 hours resulted in a reduction in the expression of cytokines such as IL-1β, IL-6, TNF-α, CCL2, IL-17, and IL-23. Furthermore, they observed that genistein inhibited STAT3 phosphorylation, IκB phosphorylation, and nuclear translocation of NF-κB in both IMQ-treated mouse skin and TNF-alpha-stimulated HaCaT cells. 41 In vitro studies conducted by Smolińska et al. revealed that treating HaCaT cells with genistein at a dose of 1 µg/mL for 24 hours suppressed the generation of reactive oxygen species (ROS) when stimulated by TNF-α or LPS. These findings suggest that in the psoriatic model, genistein has the potential to alleviate ROS-mediated NF-κB activation and NF-κB-dependent inflammatory cytokine production, particularly in keratinocytes stimulated by TNF or LPS. 42
Epigallocatechin-3-gallate (EGCG)
Green tea contains the flavonoid EGCG, whereas black tea contains tannic acid, quercetin, and the flavonoids theaflavin and thearubigin. 43 Anti-inflammatory, anticancer, antioxidant, and anti-ultraviolet radiation activities are among the advantages of EGCG that have been documented. In order to create a model of psoriasis, Zhang et al. used dosages of 0.25 g and 12.5 mg administered over a period of 6 days to IMQ-stimulated mice. 44 According to their research, local EGCG administration decreased PCNA (proliferating cell nuclear antigen) expression, which successfully prevented aberrant epidermal cell proliferation brought on by IMQ and reduced psoriasis symptoms. Moreover, the investigation showed a reduction in SOD and CAT activity following EGCG administration, indicating that EGCG may lessen mouse psoriasis symptoms via controlling antioxidant variables.
Table 1
Herbs used in treatment of psoriasis
Table 2
Emulgel formulations documented for the treatment of psoriasis.
Rutin
Rutin, a polyphenolic hydrophobic compound classified within the flavonoid family, is naturally occurring in various foods such as citrus fruits, apples, Betula leaves, buckwheat, black tea, and green tea. Renowned for its participation in antioxidant and anti-inflammatory processes, rutin has garnered attention in experimental research to evaluate its potential as an active ingredient in medicinal products. 63, 64 Derived from Memecylon malabaricum, a small tree distinguished by its blue flowers, rutin's antipsoriatic properties were investigated in in vivo studies employing a mouse tail test, as well as in vitro antipsoriatic activity using HaCaT cells at a concentration of 100 mg/mL. The research findings unveiled robust antipsoriatic activity of the plant in the mouse tail test, although it did not exhibit significant activity in any of the three conducted in vitro tests. Despite the modest activity observed in the LOX inhibition assay, Memecylon malabaricum demonstrated potent in vivo activity. These results underscore the antipsoriatic potential of the entire M. malabaricum leaf, thereby corroborating its traditional use by Siddha healers. 65
Silybum marianum
Silybum marianum, commonly known as milk thistle, is effective in treating psoriasis due to silymarin's capacity to inhibit cAMP phosphodiesterase, enhance hepatic endotoxin clearance, and prevent leukotriene production. 66 Phytochemicals present in milk thistle, including silymarin, particularly silybin, contribute to its biological functions. 67 Tea tree oil, derived from Melaleuca alternifolia, is renowned for its wound-healing capacity. The essential oil of Melaleuca alternifolia, rich in constituents like terpinen-4-ol, 1,8-cineole, α-terpineol, terpinolene, α- and γ-terpinene, is utilized for medical purposes.68
Thespesia populnea
Thespesia populnea, historically used to cure various skin conditions, including psoriasis, involves external application of oil made by boiling powdered bark in coconut oil. 69 However, there is no confirmed scientific data on its anti-psoriatic activity, prompting the selection of Thespesia populnea for scientific evidence establishment. 70 Mahonia aquifolium, also known as Barberry, contains isoquinoline alkaloids such as berberine, making it an anti-inflammatory drug used to treat skin conditions, including psoriasis. 71
Emulgels in Dermatological Applications
Emulgels represent a crucial drug delivery system, especially for administering hydrophobic active ingredients. They boast properties such as being non-toxic, non-irritating, non-sensitizing, making them highly suitable for various applications. 72 The key to formulating an effective emulgel lies in meticulously selecting the oil phase, emulsifier, and gelling agent. By choosing the appropriate emulgel excipients, optimal release of active ingredients can be achieved, facilitating their permeation through biological barriers such as the skin or intestinal mucosa. Consequently, this enhances their biological or pharmacological effects, ensuring the efficacy of the delivered therapeutic agents. 73
The controlled release mechanisms inherent in emulgels enable controlled release and targeted drug delivery. These mechanisms arise from the presence of both an emulsion and a gel system within the formulation. Various formulation factors, including the particle size of a colloidal system, surfactants, and polymers serving as gelling agents in emulgels, significantly influence the release and membrane transport of active substances. The particle size of a colloidal system plays a pivotal role in shaping the release profile of substances encapsulated within the particles. Smaller particle sizes facilitate enhanced release and penetration of active substances through the skin. Consequently, careful manipulation of these formulation factors allows for precise control over drug release kinetics and targeted delivery, ensuring optimal therapeutic outcomes. 74, 75, 76
Gelling agents in emulgels improve the stability of emulsions by boosting the viscosity of the continuous phase, leading to a slower release of active ingredients. Higher drug loading speeds up release rates, along with the inclusion of penetration enhancers in emulgels. Emulgels serve as essential carrier systems, especially for delivering hydrophobic active ingredients. Vesicular lipid systems offer a key advantage by accommodating both hydrophobic and hydrophilic drug molecules within them.
Gelling agents
Gelling agents are incorporated into a suitable medium where they dissolve or disperse, forming a weakly cohesive three-dimensional structural network with a high degree of cross-linking, achieved either physically or chemically. This process results in the production of semisolid systems. These agents are categorized into natural, synthetic, and semi-synthetic based on their origin. Natural gelling agents offer notable biocompatibility and biodegradability, although they are susceptible to microbial degradation. Examples include bio-polysaccharides or their derivatives, as well as proteins. 77, 78 In contrast, emulgel formulations commonly utilize various types of semi-synthetic and synthetic gelling agents. Semi-synthetic gelling agents exhibit superior stability and resistance to environmental influences compared to their natural counterparts. 79
Emulsion gels often utilize natural gelling agents like soy protein, whey protein, pectin, carrageenan, among others, for delivering functional food ingredients. Soy protein isolate (SPI) emerges as a favorable alternative to animal-based protein owing to its cost-effectiveness, nutritional benefits, and excellent functional attributes. Nonetheless, SPI alone may not yield gels with optimal stability properties, prompting the addition of polysaccharides to enhance gel characteristics. Wheat bran cellulose proves particularly effective in enhancing the gel properties of SPI, facilitating the development of formulations with desirable functional attributes. 9, 10
In recent times, there has been considerable interest in alginate-based food emulgels, which are created through the ionic cross-linking of alginates with divalent cations, predominantly calcium. Alginates have been combined with both traditional and protein-based emulsifiers in emulgel formulations. The gelation process and the formation of emulsion gel beads are influenced by the concentration of alginate, as well as the concentrations of protein and the oil phase. These factors can consequently impact the encapsulation, stability, and release of the encapsulated hydrophobic active substances.
Conclusion
The therapeutic landscape for psoriasis is evolving, with the development of polymeric emulgels as drug carriers promising to address limitations and meet diverse needs. The complexity of psoriatic lesions necessitates tailored treatment strategies. Emulgels offer versatile platforms for developing novel formulations like emulgels. As psoriasis prevalence increases, further research into polymeric gels is needed to enhance treatment outcomes. Understanding texture and rheological properties can improve treatment effectiveness and patient tolerability. Prioritizing formulations that meet patient preferences can optimize treatment outcomes and improve the quality of life for psoriasis patients.
