In the fast-evolving landscape of the pharmaceutical industry keeping data integrity is paramount to ensuring the safety, quality, and efficacy of pharmaceutical products. In order to prevent and identify data integrity (DI) concerns, A flexible strategy based on risk assessment that primarily depends on process comprehension and knowledge management is supported by cGMP regulations. The easy identification of mistakes, omissions, and inconsistencies throughout the data life cycle would be made possible by an efficient system architecture and controls. Several regulatory actions, including the issuing of warning letters, import warnings, and consent decrees, have been brought about by DI violations of cGMP.
According to data integrity, which is a crucial component of GMP requirements and a critical issue in any pharmaceutical health care business quality system that must guarantee that medications are of the necessary quality, data accuracy and consistency throughout the data's lifecycle are essential. As technology continues to advance the reliance on data-driven processes has increased, making it imperative for companies to adopt robust systems and practices to safeguard data integrity throughout the product lifecycle.
Data integrity gives the assurance that records i.e., electronic and paper records are precise, accurate, and well maintained in their original form and aims to prevent fabrication of information. Data integrity requirements apply equally to manual and electronic data. Data integrity awareness is crucial for several reasons across several industries, including pharmaceuticals. Here are some key reasons why organizations prioritize and promote data integrity awareness-
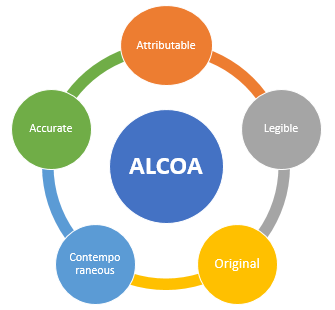
Data Integrity and ALCOA Principles
Since the 1990s, the acronym ALCOA has been in usage. Authorities of regulations have always been concerned about data integrity and ALCOA principles, particularly in relation to pharmaceuticals.
By using mathematical formulas to analyze data and associated metadata, the ALCOA set of principles ensures data integrity in a quantifiable manner. The U.S. Food and Drug Administration introduced it, and people still use it all around the world. Most often, legislative authorities demand that adherence to these standards be followed. The characteristics of the data that need to be assessed are described by these principles. The U.S. Food and Drug Administration has established clear guidelines for ALCOA+ to ensure that the data it provides is reliable, consistent, durable, and readily available.
Attributable
The information created during the activity, documented, and kept in a way that makes it possible to identify the person in charge of it.
Legible
Data on paper and in electronic form must be readable, preserved, and available at all times during the data lifetime.
Contemporaneous
To create or record data on paper or in electronic format at the appropriate moment that is accurate, whole, and reflects process observation.
Original
Data must be original rather than copies. When copies are used for audits, the original mediums must be available.
Accurate
Data must be as closest as possible to the reality and must not contain errors. No techniques that can cause deliberate information loss must be used.
Expanding Beyond ALCOA, the "+" encircles the terms "Complete, Consistent, Enduring, and Available."
Complete: Ensuring that data has pertinent metadata for thorough documentation.
Consistent: Preserving sequence and consistency by keeping the data in the proper chronological order.
Enduring: Preserving the durability and integrity of data while it is being used and stored.
Available: Facilitating simple accessibility and authorized personnel's verification.
Data Integrity in Research
According to the NIH, "Good record keeping is necessary for good science." Maintaining accurate records helps to ensure research integrity and accountability. Instrument-collected experimental data is used to identify target chemicals initially, refine prospective new medications, and demonstrate efficacy, which is required to start pre-clinical studies. Poor data integrity and records management in research records can lead to a number of problems, including troublesome collaboration and project handoffs between research teams, unsuccessful or delayed patent filing processes, poor decisions about future investments in pre-clinical studies, and even the regulatory agencies' rejection of an Investigational New Drug (IND), New Drug Agreement (NDA), or Biologics Licensing Agreement (BLA). 1, 2, 3, 4, 5, 6, 7
Data Integrity in Clinical studies
A lot of the data collected by bioanalytical laboratories goes into Phase 1-4 clinical studies and BLA and NDA applications. Not all aspects of data integrity management in a bioanalytical laboratory are related to collecting clinical and non-clinical data. There are many other steps that need to be completed, including transportation and stability of samples and analytes, technique and instrument validation, sample and study management, documentation, and training. Regulatory organizations in each country where sponsors plan to market their products have updated rules that labs must follow.