Introduction
Emblica officinalis (amla/aonla) is a well known medicinally valued plant, the efficacy of amla fruit is widely proved, however, the use of its leaves is less investigated. Antimicrobial property of petroleum ether leaf extract of amla was tested by disc diffusion method and the MIC of antimicrobial activity, concentration ranging from 1000 μg to 62.5 μg was compared with the standard drug streptomycin (10 μg) and amphotericin B (20 μg). The study revealed that the extract has potential antibacterial activity, leaves have broad spectrum of antimicrobial activity and a potential source for new class of antibiotics that could be useful in chemotherapy / control human infectious diseases (Elangovan et al., 2015). The aqueous crude extract of amla was screened by using agar well diffusion method against five human bacterial pathogens such as Bacillus sp., Lactobacillus sp., Pseudomonas sp., Proteus sp. and Streptococcus sp. at the concentrations of 30, 60 and 90μl. The data clearly shows that the extract possesses strong inhibitory action against all the test bacterial pathogens (Kanthimathi and Soranam, 2013). In vitro antibacterial activity of aqueous, ethanolic and acetone extracts of fruit was evaluated against gram-positive vs. gram-negative bacteria employing S. aureus and E. coli respectively. All the extracts exhibited significant antibacterial activity, more against S. aureus than E. coli. Among the extracts, acetone inhibited the growth of S. aureus and E. coli at minimum concentration of the extract (0.1 μg and 1.0 μg respectively). MIC for ethanol and aqueous extracts were 0.3 and 1.0 μg, 1.5 and 3.75 μg, for S. aureus, E.coli respectively.1, 2, 3 It is concluded that E. officinalis is more inhibitory to gram-positive than the gram-negative bacteria (Varghese et al., 2013).
Extracts of different solvents of the leaf and bark of the plant were used to identify the bioactive compounds and antimicrobial activity was checked against different human pathogens (MTCC). Methanolic extract of amla showed highest zone of inhibition against E. aerogens and E. feacalis and acetate extract showed antifungal activity against Rhizomucor species (Sukanya et al., 2013). Antifungal property of E. officinalis was also reported against Aspergillus species (Satish et al., 2007). Ethanol and acetone extracts of fruit showed moderate activity against F. equiseti and C. albicans where grisofulvin was used as standard antibiotic (Hossain et al., 2012).4, 5, 6, 7, 8
Amla is also reported to possess potent free radical scavenging, antioxidant, anti-inflammatory, anti-mutagenic and immunomodulatory activities which are efficacious in the prevention and treatment of various diseases like cancer, atherosclerosis, diabetes, liver and heart diseases. Therefore, an attempt was made to assess the antibacterial property of E. officinalis using Agar–well diffusion method against some common bacterial pathogens.
In view of its medicinal and antimicrobial properties the present study aimed at assaying quantitatively the antibacterial activity of methanolic extracts of E. officinalis against gram positive and gram negative bacterial strains
Materials and Methods
Antimicrobial activity
Determination of antibacterial activity
Qualitative analysis for evaluating antimicrobial activity of test material was carried out by agar–well diffusion method (Feyza et al., 2009) with modification. Two gram positive (Bacillus subtilis, MTCCC 2389 and Staphylococcus aureus MTCC7443) and three gram negative (Micrococcus luteus, MTCC4821., Escherichia coli, MTCC2127., Klebsiella pneumoniae, MTCC7162) bacterial strains were used in the present study. 20 mL of sterilized nutrient agar was inoculated with 100 µL of bacterial suspension (108 CFU/mL) and then poured on to sterilized petri plates. The agar plates were left to solidify at room temperature.9, 10, 11, 12 A well of 6 mm diameter was aseptically bored into the agar plates and 20 µL of the essential oil (diluted with DMSO, 1:1) was added in each well. Chloramphenicol (5μg) was used as a positive reference to determine the sensitivity of bacteria and DMSO as negative reference. The plates were kept at 4 oC for 2 h to allow the dispersion and then incubated at 37 oC for 24 h.
Determination of MIC by broth dilution method
Broth dilution technique was used to determine the minimum inhibitory concentration of the test material against bacterial strains. One millilitre of nutrient broth was kept in each tube and autoclaved. The extract diluted with DMSO (1:1) was filtered with 0.22 μm filter disk before use and then added to each tube to keep the final concentration ranging from 62.5 μg/mL–2000 μg/mL. The test bacterial suspension was added into each tube to yield bacterial density of 106 CFU/mL and the inoculated tubes were incubated at 37 oC for 24 h. Tubes containing nutrient broth without essential oil served as positive control, whereas those without bacteria as negative control. After incubation, 50μL of 0.2 mg/mL p-iodonitrotetrazolium violet (INT) was added in each tube to indicate the bacterial growth. The tubes were again incubated for 30 min at 37 oC. Development of pink colour in the tube (due to reduction of dye) indicated the bacterial growth whereas tubes without colour indicated no active bacterial growth. The lowest concentration at which no bacterial growth was observed (as indicated by colour) corresponded to the minimum inhibitory concentration (MIC). All the assays were performed in triplicate.
Results and Discussion
The explore for antimicrobials from natural resources has been received much attention to identify compounds that can act as suitable antimicrobials agent to replace synthetic products Phytochemicals derived from plant products serve as a prototype to develop less toxic and more effective medicines in controlling the growth of microorganism (Kelmansone et al., 2000) and (Ahmad et al., 2001). These compounds have significant therapeutic application against human pathogens including bacteria, fungi or virus. Numerous studies have been conducted with the extracts of various plants, screening antimicrobial activity as well as for the discovery of new antimicrobial compounds (Guleria et al., 2006) and (Zakaria et al., 2007). The present study was carried out to evaluate in vitro antibacterial potential of methanolic extract of Emblica officinalis leaves. The antibacterial activity was screened by using Agar-well diffusion method against two Gram positive strains viz., Bacillus subtilis MTCC2389, Staphylococcus aureus MTCC7443 and Gram negative strains like Escherischia coli MTCC212 and Klebsiella pneumoniae MTCC7162 and Micrococcus luteus MTCC4821.
Table 1
Antibacterial analysis of Emblica officinalis leaves
Figure 1
Antibacterial activity of Emblica officinalis extract against A:) Klebsiella pneumonia and B:) Micrococcus luteus by agar well diffusion assay C:) Staphylococcus aureus and: D:) Bacillu subtilis by agar well diffusion assay
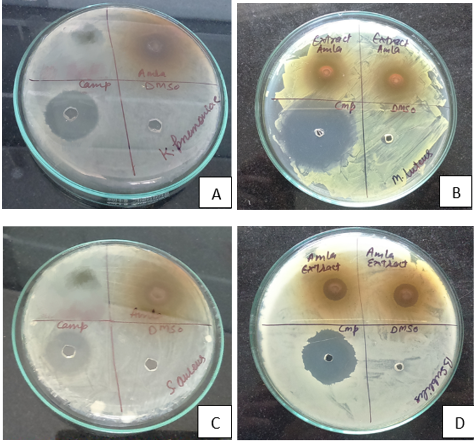
The extract showed maximum zone of inhibition against Micrococcus luteus (17.6mm) followed by Klebsiella pneumoniae (16.5mm) and Bacillus subtilis (16.2 mm) and lowest against Staphylococcus aureus (15.5mm) and Escherischia coli (15mm) (Table 1). The present study also revealed that the methanolic extracts of amla exhibited significant antibacterial activity more against Micrococcus luteus than others bacterial strains .The different concentrations of extract were used for this tests were 10μl and 20μl.The minimum inhibitory concentration (MIC) of antimicrobial activity of the amla extract at a concentration ranging from 2000 μg to 62.5 μg was compared with the standard drug Chloramphenicol (disc 5μg). The antibacterial activity exhibited by the E. officinalis could be attributed to the presence of bioactive components namely flavonoids, phenols, saponins, tannins in the plant Javale and Sabnis (2010). The results of present study revealed that leaves of amla contain high antibacterial potential and source of new classes of antibiotics that could be useful in chemotherapy and control on human infectious diseases.
Conclusion
The present study disclosed the importance of natural medicinal plant extract to control pathogenic bacteria which pose threat to human health and it also concludes that leaves of amla contain high antimicrobial properties. The presence of phytochemicals like flavonoids, tannins, saponins glycosides and phenolics compounds are major constituents in Embilica officinalis which may acknowledge the medicinal property of this plant. This implies that the methanolic extract may indeed be effective in the management of diseases caused by these encountered pathogens and supporting its ethno medicinal uses and this plant also safe, potent and cost effective to treat many infectious diseases of livestock, poultry and human. There is need for further investigation of this plant in order to identify and isolate its antimicrobial agent.
