Introduction
We are continuously exposed to a number of pollutants that generate free radicals in our body and can cause various diseases.1, 2 Plants produce a number of phytochemicals that have many therapeutic benefits, and regulate metabolic processes and maintain good health.3 It has been established that oxidative stress is one of the major causes of many chronic and degenerative diseases including, heart disease, ageing, diabetes, cancer, immune-suppression, and neurodegenerative diseases. 4, 5 Oxidative processes are the main routes for producing free radicals (reactive oxygen species/ reactive nitrogen species) in living systems.6 Reactive oxygen species (ROS) can damage macromolecules like lipids, nucleic acids, and protein which may lead to functional disorders.7 The most efficient pathway to eliminate and diminish the action of free radicals, which cause oxidative stress, is antioxidative defense mechanisms. Antioxidants are substances that possess free radical scavenging properties. Recently, there have been increases of interest in medicinal plants having therapeutic potential as antioxidants to reduce oxidative stress-induced tissue injury.8
A large number of aromatic and medicinal plants contain bioactive compounds exhibiting high antioxidant properties. Cassia fistula (CF) is an important medicinal plant belonging to the family Caesalpiniaceae. The whole plant has various medicinal properties useful in the treatment of skin diseases, inflammatory diseases, rheumatism, anorexia, and jaundice.9 Among the numerous naturally occurring antioxidants; ascorbic acid, carotenoids and phenolic compounds are more effective.10 They are known to inhibit lipid peroxidation (by inactivating lipoxygenase), to scavenge free radicals and other ROS by propagating a reaction cycle and to chelate heavy metal ions.11 The study conducted on medicinal plants strongly supports the idea that plant constituents with antioxidant activity are capable of exerting protective effects against oxidative stress in biological systems.12 In our experimental work, we studied about quantification of phytochemicals and evaluation of antioxidant activity in different parts of Cassia fistula plant. The phytochemicals were estimated in different plant parts, and antioxidant activities were carried out during the course of the work. Total phenolic content was also correlated with different antioxidant studies. The assessment of such properties remains an interesting and useful task, particularly for finding new sources for natural antioxidants for food and pharmaceutical companies.
Materials and Methods
Plant materials
Samples were collected from the campus of Dr. Rammanohar Lohia Avadh University, Ayodhya, India, and plant identification was done by Dr. MN Srivastava, Botany Division, CSIR-Central Drug Research Institute, Lucknow, India (14812 CSIR-CDRI). The collected samples were washed two times with tap water, dried, powdered, and stored in polythene bags at 4oC till further processing.
Chemicals and reagents
Bovine serum albumin (BSA), gallic acid, and quercetin were obtained from Sigma-Aldrich, St. Louis, USA. Folin Ciocalteu's phenol reagents, ascorbic acid, and β-carotene were purchased from E-Merk, Mumbai, India. 2,2-diphenyl-1-picrylhydrazyl (DPPH), reduced nicotinamide adenine dinucleotide (NADH), nitro blue tetrazolium (NBT), thiobarbituric acid (TBA), potassium ferricyanide, trichloroacetic acid (TCA), phenazine methosulphate (PMS), sodium dodecyl sulfate and ferric chloride products were from SRL, India. Other chemicals used in the experiments were of analytical grade. Preparation of solutions was carried out in de-ionized ultrapure water (Direct Q5, Millipore, Bangalore, India).
Estimation of phytochemicals
Ascorbic acid content of C. fistula leaf (CFL), seed (CFS) and bark (CFB) extracts was assessed by the AOAC method and data were reported as mg/100g of fresh weight (FW) of tissues.13 Estimation of carotenoids (in μg/g of FW) was carried out by the method of Jensen.14 Total phenolic content (TPC) measurement was performed using the Ragazzi and Veronese method and values were reported in mg of gallic acid equivalent (GAE)/g of dry weight (DW).15 Estimation of protein content was done by Lowry method16 whereas carbohydrate content was assessed by the anthrone method and both values were reported as mg/g of DW.17
Extraction procedure
Dried 20 gram powdered plant samples of C. fistula leaf (CFL), seed (CFS) and bark (CFB) were extracted with 70% ethanolic solvent for overnight at room temperature in a rotatory shaker. The extracted solvents were filtered through with Whatman No.1 filter paper. The filtrates were re-extracted untill discoloration. The pooled extracts were concentrated at 40oC using a rotary evaporator and lyophilized untill crude extracts were dried. Powdered crude extracts were kept at -4oC.
Antioxidant studies
Estimation of TPC in crude extract was also performed with the Ragazzi and Veronese method.15 The TPC was described as mg of gallic acid equivalent (GAE)/g of crude dry weight. DPPH (1,1-diphenyl-2-picrylhydrazyl) dye was used to determine the free radical scavenging activity (FRSA) of samples based on the method described by Yen and Duh18 whereas superoxide anion radical scavenging activity (SARSA) was assayed with the method of Nishikimi et al.19 which was based on the reduction of nitro blue tetrazolium (NBT). Plant extract’s reducing power (RP) was assayed by the Apati et al. method.20 Lipid peroxidation assay was performed by using egg homogenates as lipid rich media according to Ohkawa et al.21 Hydroxyl radical scavenging activity (HRSA) was evaluated by the method of Halliwell et al.22 through hydroxyl radicals generated by a mixture of Fe3+-EDTA, H2O2 and ascorbic acid. Ferric thiocyanate assay (FTC) was determined by testing the inhibitory capacity of extracts against the oxidation of linoleic acid by Tsuda et al.23
Statistical analysis
Results were reported as mean ± SD (standard deviation) of three replications (n=3). Data were analysed on PRISM software using the student’s t-test and one way ANOVA. To show the significant results, * p≤0.05; ** p≤0.01 and *** p≤0.001 were considered as levels of significance.
Results
Phytochemicals estimation
The highest ascorbic acid content was present in CFL (220.34 mg/100g of FW) followed by CFS and CFB. CFL showed the highest concentration of carotenoids (16.56μg/g of FW), whereas CFB showed the lowest concentration (1.25 μg/g of FW). Results showed that CFB had the highest TPC (101.9 mg/g of GAE of DW) and protein content (176.6 mg/g of DW) in comparison to CFL and CFS. The maximum carbohydrate content was found in CFS (95.54 mg/g of DW) followed by CFL (62.22 mg/g of DW) and CFB (52.22 mg/g of DW) (Table 1).
Table 1
Quantification of phytochemicals in different parts of Cassia fistula
Antioxidant studies
Total phenolic content (TPC)
Total phenolic content estimated in ethanolic extracts of CFL, CFS and CFB was 74.65, 67.78 and 114.23 mg/g of GAE of DW, respectively (Figure 1). The highest TPC value in CFB displayed its high antioxidant potential in comparison to CFL and CFS.
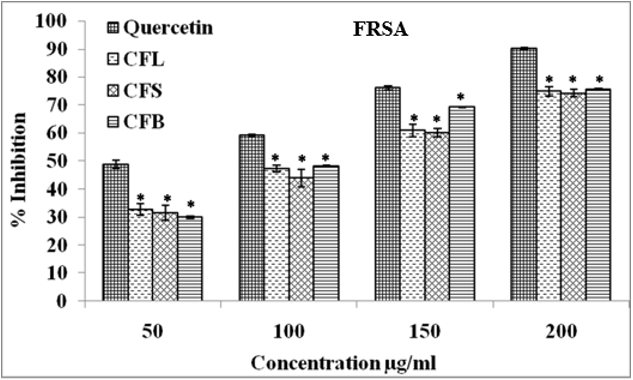
Free radical scavenging activity (FRSA)
Free radical scavenging activity in different parts of Cassia fistula was determined with the reduction of DPPH. Plant extracts reduce the DPPH free radicals and make them stable and colourless.24 Result showed that the CFB extract had the most effective free radical scavenging activity compared to CFL and CFS. The tested plant samples showed concentration dependent (50-200 μg/ml) DPPH reductions ranging from 29.99 to 75.62% (Figure 2). FRSA potential of tested samples and standard at 200 μg/ml, was in the following order: CFB (75.62%) > CFL (75.02%) > CFS (74.16%) in contrast to standard quercetin (90.25%). The minimum inhibitory concentration (IC50) value of CFB was 35.48 μg/ml whereas the IC50 values for CFL and CFS were 36.81 and 38.48 μg/ml, respectively.
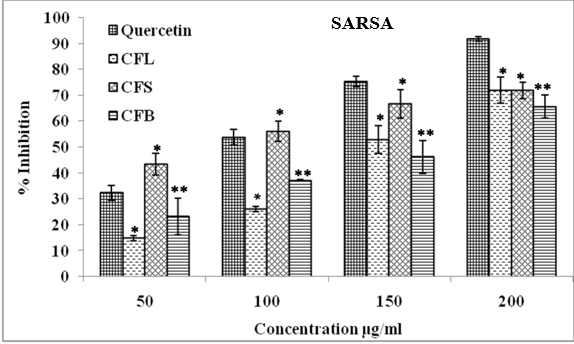
Superoxide anion radical scavenging activity (SARSA)
Superoxide anion radical scavenging activity was estimated by NBT reduction which showed that CFL had the highest inhibition of NBT reduction followed by CSF and CFB. The CFL extract inhibited the production of superoxide anion radicals by 14.86, 26.03, 52.90 and 72.06%, respectively, when 50, 100, 150, 200 μg/ml of plant extract concentrations were applied to the reaction mixture. The SARSA of tested plant samples at 200 μg/ml was in the following order: CFL (72.06%) > CFS (71.90%) > CFB (65.63%) in comparison to quercetin (91.77%) (Figure 3).
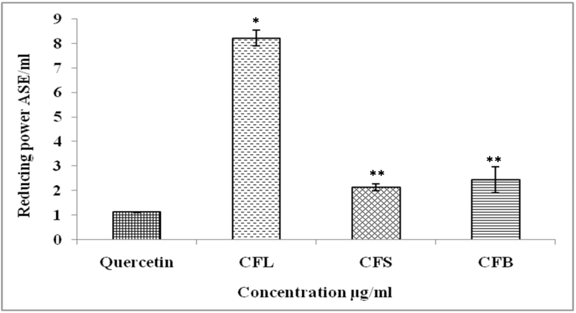
Reducing Power (RP)
The CFS extract had significantly higher Fe3+ to Fe2+ reduction potential (2.14 ASE/ml) as compared to CFL (8.22 ASE/ml) and CFB (2.44 ASE/ml) whereas quercetin (1.12 ASE/ml) had the maximum reduction potential in comparison to tested plant samples (Figure 4).
Lipid peroxidation
Anti-LPO activity of plant extracts was carried out using egg homogenate as a lipid-rich source and measured in terms of MDA formation. The ethanolic extracts of CFL, CFS and CFB inhibited MDA formation and hence reduced lipid peroxidation. Maximum anti-LPO activity was shown by CFB in a concentration dependent manner (100-400 μg/ml). Anti-lipid peroxidation potential of tested plant samples was as follows: CFB > CFL > CFS. Standrad quercetin at 400 μg/ml concentration showed maximum scavenging activity (85.39%) as compared to tested plant samples CFS (70.36%), CFL (72.80%) and CFB (75.70%) (Figure 5).
Hydroxyl radical scavenging activity (HRSA)
The antioxidant activity of C. fistula plant extracts was further studied for its scavenging potential of hydroxyl radical (OH•) by using deoxyribose degradation assay. The CFB was found to have the highest OH• scavenging activity among the tested plant samples and showed 48.70 to 58.30% scavenging activity with increasing extract concentration (50-200 μg/ml). Quercetin had the maximum hydroxyl radical scavenging activity (91.12%) at 200 μg/ml concentration. Based on their OH• scavenging potential, the antioxidant activity was as follows: CFB > CFS > CFL (Figure 6).
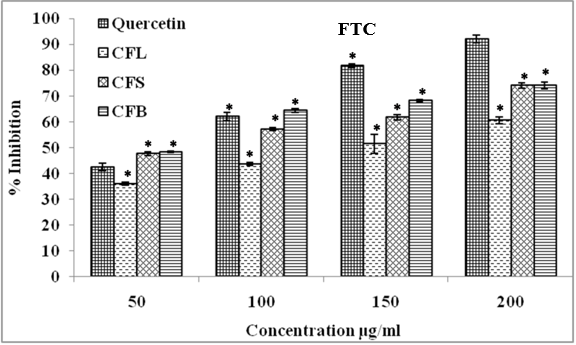
Ferric thiocyanate assay
Ferric thiocyanate assay is used for the determination of the presence of lipid hydroperoxides formed due to ferrous ions and to evaluate the antioxidants effects of plant extracts. The scavenging activity improved with increasing plant extract concentrations from 50 to 200 μg/ml in the reaction mixture. Among the tested extracts, CFB had maximum inhibition (74.10%) followed by CFS (74.06%) and CFL (60.60%) whereas quercetin had 92.12% inhibition to obstruct peroxide production at a 200 μg/ml concentration (Figure 7).
Discussion
Ascorbic acid plays a very important role as a primary antioxidant in plasma as well as in cells and enhances the body’s antioxidant defense. 25, 26 It reduced the reactive oxygen species formation and their reduction to improve the body’s antioxidant defense or delay in the disease’s onset.27 Carotenoids are powerful free radical scavengers and also provide support to the body's immune system against infections. Epidemiological studies have shown that a high carotenoids rich diet intake is associated with reduced cardiovascular incidence as well as degenerative diseases. The carotenoids protective role in the body is to reduce the antioxidant enzymes degradation because of singlet oxygen deactivation.28 Carotenoids showed a direct link between enhancement of the immune system and decreased risk of degenerative diseases.29, 30 TPC is mostly responsible for the antioxidant activity in plants. It has been reported that high antioxidant activity is directly associated with the presence of high phenolic content in plant samples.31 According to Singh et al. proteins have excellent potential to scavenge free radicals/ROS. 32 Proteins displayed anti-LPO activity through ROS inactivation as well as peroxidative transition metal chelation. Protein isolated from Phyllanthus niruri L., shows a hepatoprotective role against CCl4 induced liver toxicity through its antioxidant properties.33 The plant M. charantia contains a hypoglycaemic protein called polypeptide-p, which is used to control diabetes naturally and works similarly to human insulin.34 Among numerous natural antioxidants, polysaccharides in general have strong antioxidant activities and can be explored as novel potential antioxidants.35
Reported literature shows that phenolic compounds have a high reduction potential, which allows them to act as antioxidants.36 As their free radical scavenging ability is facilitated by their hydroxyl groups, the TPC could be used as a basis for rapid screening of antioxidant activity. Therefore, plants containing high level of phenolic compounds could be a great natural antioxidant source. Studies reported in the literature on plant extracts antioxidant activity suggest that there is a linear correlation between phenolic content and antioxidant activity.37 In earlier studies by Genwali et al. 38 reported that the methanolic extract of C. fistula leaf had 138.63 mg/g of GAE TPC which is lower than the ethanolic extract value reported by us (174.65 mg/g GAE). The result reported by us showed agreement with a study by Taso and Deng 39 which also confirmed that phenolics are usually better extracted by using alcohol solvents than methanol.
DPPH is stable nitrogen containing synthetic radical. DPPH method is a preferred method for assaying the free radical scavenging activity of plant extracts because it is fast, easy and reliable and does not require a special reaction or device. The DPPH free radical is a colourful dye that receives an electron from antioxidant bioactive compounds to become a stable molecule with stabilize themselves in to a sable molecule and become colourless. Discoloration occurs (from purple to yellow) due to the decreasing quantity of DPPH free radicals in the reaction mixture. The discoloration potential of plant extracts reflects the radical scavenging activity of the analysed extract. CFB showed the lowest IC50 value of 35.48 µg/ml followed by CFL (36.81 µg/ml) and CFS (38.48 µg/ml). El-Hashash et al. 40 also reported that CFL methanolic extract significantly scavenges free radicals and has a 132.86 μg/ml IC50 value.
The superoxide anion is the primary reactive oxygen species and is connected with regulation of inflammatory pathways. The superoxide anion was assayed with the help of PMS‐NADH‐NBT system and reduction in absorbance at 560 nm with plant extracts indicates its antioxidant capacity. From the reported results, it has been found that plant extracts showed concentration dependent free radical scavenging capacity in tested antioxidant parameters. It was established that plant samples containing antioxidant molecules are able to prevent the formation of blue formazan complex.41 Earlier studies by Kalaiyarasi et al.42 showed SARSA of ethyl acetate, hexane and aqueous extracts of C. fistula pods had IC50 values of 51.38, 53.23 and 440.52 μg/ml which were higher than the values reported by us for ethanolic extracts of CFS (24.54 μg/ml) and CFB (49.86 μg/ml). This difference in result may be due to higher concentration of phenolic compounds in ethanolic extract than in other solvents.
In the RP assay, with the addition of plant extracts to the reaction mixture, the colour of the reaction mixture changed from yellow to various shades of green and blue depending on the reducing potential of compounds present in plant extracts. The presence of reducers causes the conversion of the Fe3+/ferricyanide complex used in this method to ferrocyanide which then reacts with FeCl3 to form a blue coloured complex (Perl’s Prussian blue). By measuring the formation of Pearl’s Prussian blue at 700nm, it is possible to determine the concentration of the Fe3+ ion. The reducing power of plant extracts may serve as a significant indicator of their potential antioxidant activity.43 An earlier study by Irshad et al.44 showed the strong RP potential of methanolic (2.5 ASE/ml) and hexane (5.00 ASE/ml) extracts of C. fistula seed, which is in agreement with the current study. Our data on the RP of the C. fistula bark and seed extracts suggest that it has a comparable antioxidant effect in comparison to standard quercetin.
Lipid peroxidation is a prominent indicator of oxidative stress in cells and tissues or cellular injury in both animals and plants.45 In the LPO assay, the peroxidation of membrane lipids was induced by FeCl3 and ascorbic acid as reducing agents. OH• is generated by mixing Fe3+ and ascorbate which attack the biological molecules. Lipid peroxidation leads to the formation of MDA and other aldehydes, which react with thiobarbituric acid to form pink chromogen thiobarbituric acid reactive substances (TBARS) with TBA, absorbing at 532 nm. The production of aldehyde is used as a biomarker to measure the level of oxidative stress in an organism.46 According to previous research findings, which showed aqueous and methanolic bark extracts of C. fistula exhibited 40.52% and 64.12% anti-LPO activity at 400 μg/ml concentration in comparison to our reported inhibition potential of bark ethanolic extract (75.70%).47 The high anti-LPO activity of CFB may be attributed to its higher content of phenolic compounds. Consequently, the LPO inhibition could be caused by the chelation of Fe2+or by trapping free radicals.48
OH• is the major active ROS, causing lipid peroxidation and enormous biological damage. In the HRSA assay, hydroxyl radical produced by the Fenton reaction oxidised 2-deoxy-2-ribose sugar and had an open circular structure. The oxidative degradation can be noticed by heating the products with TBA under acidic conditions to grow a pink chromogen (thiobarbituric acid reactive species) with a maximum absorbance at 532 nm. Extracts that are tested, compete with deoxyribose and reduce chromogen formation in a dose-dependent manner. Ilavarasan et al.47 stated that the aqueous and methanolic CFB extract displayed 45.21 and 56.30% inhibition against OH• at 200 µg/ml concentration, which is lower in comparison to the ethanolic extract of bark (58.30%) reported by us.
FTC assay is used to measure the early stage of lipid peroxidation which produces different peroxides. In this assay, ammonium thiocyanate is used as a colouring reagent that binds with iron and reduces lipid peroxidation. Our experimental results explored that CFB extract act as an active scavenger of Fe3+ ions and showed similar results reported by other studies. 48 The experimental results also showed that increasing plant extract concentration reduces peroxide formation. Based on these data, ethanolic extracts of C. fistula plant parts may be a good source for nutraceuticals that are capable of acting as natural antioxidant.
Conclusion
It is well known that ROS are involved in the development of various chronic diseases such as cardiovascular, neurodegenerative, cancer, ageing, atherosclerosis, and inflammatory diseases. Our study revealed that among the different parts of C. fistula, CFB had high total phenolics and protein content whereas CFL had high ascorbic acid, carotenoids and carbohydrate content. Exploration of antioxidant activity in different parts showed that CFB had maximum antioxidant activity in terms of IC50 values for FRSA, LPO, HRSA and FTC, whereas CFS showed the maximum antioxidant activity for SARSA and highest RP. The antioxidant activities of C. fistula may be credited to their strong hydrogen donating and metal chelating abilities, as well as effective free radical scavenging activity. We can infer from the obtained results that the bark of C. fistula plant had higher phytochemical constituents and higher antioxidant activity which may serve as a good source of nutraceuticals and natural antioxidant in food as well as for pharmaceutical companies.