Introduction
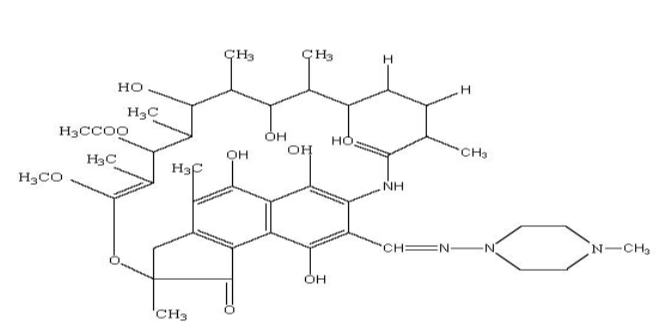
Rifampicin is an antibiotic used for the treatment of tuberculosis and other infectious disease derived from streptomyces mediterranei.1 Tuberculosis is nothing but a deadly infectious disease that is responsible for two million people death each year. Rifampicin is treated as the key to the current treatment of tuberculosis due to its active sterilizing ability.2 Rifampicin is an antimicrobial drug for Mycobacterium tuberculosis.3 In order to ensure its quality, International Pharmacopoeia (IP), British Pharmacopoeia (BP), United State of Pharmacopoeia (USP) narrated analytical procedure.4, 5, 6 According to the outline of literature survey, high performance liquid chromatography (HPLC), high performance thin layer chromatography (HPTLC), visible spectrophotometry method available for the quantification of Rifampicin (Figure 1: molecular structure of Rifampicin) in pharmaceutical solid dosage form.7
The purpose of this study is to develop and validate a stability indicating reverse phase high performance liquid chromatography (RP-HPLC) for assay quantification of Rifampicin in pharmaceutical solid dosage form.
Materials and Methods
Instrument and chromatographic condition
The chromatographic condition was performed into waters Alliance HPLC 2695 system with PDA detector (USA) connected with empower 3 software; column: 4.6 x 250 mm inner diameter (ID), 5µm with particle size (Phenomenex Luna); flow rate: 1.0 ml/min; injection volume: 10µL, detector: uv 254 nm with run time 15 minutes.
Chemicals, reagent and standard
Rifampicin working standard (potency: 100.0%) used of Olon Active Pharmaceuticals manufacturer, acetonitrile and methanol used for HPLC grade of Scharlau manufacturer, sodium hydroxide and sodium acetate trihydrate used for analytical grade of scharlau manufacturer, glacial acetic acid used for analytical grade of Merck manufacturer.
Method development
Based on solubility of Rifampicin (at pH 7.06 solubility of Rifampicin 0.85 mg/ml),8 maximum absorbance, pKa value of Rifampicin (Zwitterions with pKa 1.7 related to the 4-hydroxy and pKa 7.9 related to the 3-piperazine nitrogen),9 molecular weight of Rifampicin and polarity of Rifampicin; detection and quantification reverse phase high performance liquid chromatography (RP-HPLC) method was optimized. The proposed detection and quantification technique was; mobile phase (60% acetate buffer pH 4.5 with 40% acetonitrile), diluent (60% phosphate buffer pH 7.0 with 40% methanol), wavelength 254 nm.
Preparation of Standard Solution
Accurately taken 20 mg of Rifampicin working standard into 100 mL volumetric flask. Added 60 ml of diluent with sonication for 10 minutes. Cooled to room temperature. Diluted up to the mark with diluent. Concentration 0.20 mg/ml
Preparation of sample solution
Taken 20 tablets and crushed them uniform. Accurately taken 200 mg equivalent of Rifampicin into 1000 ml volumetric flask. Added 200 ml of diluent and mechanically shaked at 200 rpm for 10 minutes. Added 600 ml of diluent to the same volumetric flask and sonicated for 15 minutes with intermittent shaking. Cooled to room temperature and diluted up to the mark with diluent. Centrifuged the solution at 7000 rpm for 3 minutes. Taken the supernatant solution for HPLC vial. Concentration: 0.20 mg/ml
Method validation
According to ICH Q2R1 analytical method validation guideline, this method was validated considering system precision, specificity, linearity, accuracy, method precision and intermediate precision.10
System precision
System precision of reverse phase high performance liquid chromatography (RP-HPLC) was confirmed by five replicate injection of standard solution for Rifampicin. The main objective of system precision was to identify whether the system for analysis was ready or not. Figure 2 for representative chromatogram of standard solution for Rifampicin.
Specificity
In order to conduct specificity study, Rifampicin quinone impurity 0.005 mg/ml, placebo, blank and test solution was run into the HPLC system. The aim of this study was to detect any interference available or not for impurity, blank and placebo at the elution zone of Rifampicin and also to confirm the peak purity of Rifampicin in test solution. Figure 3, Figure 4 for impurity and peak purity chromatogram.
Linearity
Linearity was estimated by injecting five standard solution ranges from concentration 0.15 mg/ml to 0.30 mg/ml covering 50% to 150% with respect to 100% standard concentration 0.20 mg/ml. Figure 5 for calibration curve of linearity study.
Method precision
Six independent sample solutions were prepared for method precision study using waters (USA) PDA reverse phase high performance liquid chromatography (RP-HPLC). Table 3 tabulated the method precision study results.
Intermediate precision
Intermediate precision study was executed in different day using six different sample solutions in Agilent (USA) PDA reverse phase high performance liquid chromatography. Table 3 for intermediate precision result.
Accuracy
80%, 100% and 120% of Rifampicin spiked solution was prepared for 9 determinations to calculate the accuracy of the analytical method. Table 3 showed the results for accuracy results.
Limit of quantification
Diluent solution of standard solution was prepared to calculate the sensitivity of the method based on signal to noise ration. On 0.18 ppm concentration of Rifampicin standard solution the signal to noise ration observed 47. So, the sensitivity of the method claimed for 0.18 ppm.
Results and Discussion
Specificity
The method was specific and sensitive for Rifampicin peak detection and peak purity of Rifampicin passed. No interference observed at the elution zone of placebo, blank and impurity solution. Purity angle of Rifampicin was 0.066 and purity threshold was 0.225 for test solution.
Linearity
Table 2
Correlation coefficient and regression analysis
|
Linearity range (mg/ml) |
0.10 mg/ml to 0.30 mg/ml |
|
Regression equation (y=mx +c) |
36077x - 4207 |
|
Slope (m) |
36077 |
|
Intercept (c) |
-4207 |
|
Correlation coefficient, R2 |
1.000 |
Method precision and intermediate precision
Accuracy?
Table 4
Nine determinations of accuracy results
Conclusion
Based on above analytical data, it can be concluded that this reverse phase high performance liquid chromatography (RP-HPLC) method is stability indicating, sensitive, specific for Rifampicin peak detection and quantification, precise and accurate for assay estimation in pharmaceutical solid dosage form.