- Visibility 34 Views
- Downloads 3 Downloads
- DOI 10.18231/j.ijpca.2023.005
-
CrossMark
- Citation
A review on specific and sensitive analytical method development and validation
- Author Details:
-
MD Nazmus Sakib Chowdhury *
Introduction
Specific and sensitive analytical method development is the prerequisite of method validation. It involves analytical chemistry that deals with quantification and qualification of target molecules.[1] Specific and sensitive analytical method development is required for noncompendial method. Based on molecular nature and physiological properties of target molecule, critical quality attributes of the method selected. After the selection of analytical method, method validation conducted. Based on method validation report, analytical test procedure has updated. Specific and sensitive analytical method development is the continuous process of solving problems finding at any site of stage of product development.[2] The analytical chemistry and both quantification and qualification of target molecule conducted based on current good manufacturing practices (cGMP) and USFDA (Food and drug administration, USA) regulations.[3]
Discussion
Sensitive & specific analytical method development
Analytical method development introduces a procedure for unknown compound identified and quantified from sample matrix. When no detection technique available in compendia, specific molecule method development needs to initiate based on molecular nature, pH, pKa and other physicochemical properties. Based on method development, method validation conducted for the target molecule. However, any problem finds at the time of routine use of that analytical method, method development initiates and revalidated.[4]
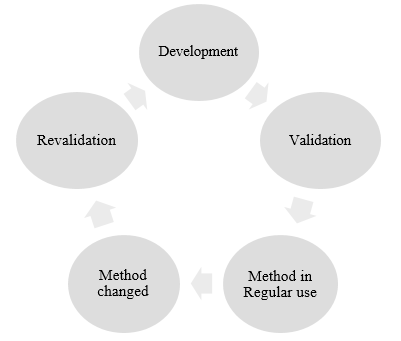
Analytical Method Validation
The goal of analytical method validation is to produce evidence that the method is suitable for its intended purpose. Validation involves Accuracy, Specificity, Method precision, Intermediate precision, Linearity, Limit of detection (LOD), Limit of quantitation (LOQ) and Robustness.
Accuracy
The accuracy of an analytical procedure is the closeness of agreement between the value which is accepted either as conventional true value or an accepted reference value and the value found. The accuracy of method can confirm by analyzing 9 determinations covering three different concentration of triplicate sample.
Accuracy of prochlorperazine maleate (PRO) and betahistine hydrochloride (BET) was confirmed by the addition of standard solution among three different concentration for triplicate samples (50%, 100%, and 120%). A defined amount of drug was added to the test sample and percentage recovery calculated. When this method was used for accuracy, the recovery was found to be 99.5% for betahistine hydrochloride and 99.7% for prochlorperazine maleate.[5]
Method Precision (Repeatability)
The method precision is studied by preparing six different sample solutions of 100% concentration.
The method precision of prochlorperazine maleate (PRO) and betahistine hydrochloride (BET) was confirmed by the addition of standard solution by repeated injection (n = 6) of BET (15μg/ml) and PRO (15μg/ml). The % RSD found for six repeated injection of standard solution is 0.227% for prochlorperazine maleate (PRO) and 0.219% for betahistine hydrochloride (BET).[5]
Limit of detection and limit of quantitation
Limit of detection is the lowest amount required to detect the analyte by specific and sensitive analytical method and it can be calculated based on calibration curve
LOD = 3.3 x ϭ/S
Where,
ϭ = Standard deviation of the response obtained from calibration curve
S = Slope of calibration curve
Limit of quantitation is the lowest amount required to quantify the analyte by specific and sensitive analytical method and it can be calculated based on calibration curve
LOQ = 10 x ϭ/S
Where,
ϭ = Standard deviation of the response obtained from calibration curve
S = Slope of calibration curve
Linearity study
Linearity study performs by preparing at least five different concentrations by dilution of the standard stock solution.
The linearity study of paracetamol was performed by preparing 5 different concentrations among (6.25, 12.5, 25, 50, and 100μg/mL) and found the correlation coefficient between concentration 6.25 to 100 μg/mL is 0.999.[6]
Intermediate precision
Intermediate precision study performs by a typical variation from method precision (Instrument, different day, different analyst, different column etc.)
Specificity
Specificity confirms whether the method is specific and sensitive for the target analyte or not. In specificity, there should be no interference at the elution zone of the target analyte for blank, placebo or other degradants.
Specificity was conducted to identify the elution zone of each drug in a mixture and in the sample. The elution zone of standard drugs individually was determined, and it was found to be 3.750 min and 1.533 min for nitazoxanide and elution zone of both drugs in the standard mix was found to be 3.760 min for nitazoxanide and 1.542 min for ofloxacin respectively.[7]
Conclusion
This article provides information regarding method development to method validation. Method validation is an integral part of pharmaceutical manufacturing company for the support of production. It confirms product quality. So, specific and sensitive analytical method is a must for pharmaceutical product quality assurance.
Source of Funding
None.
Conflict of Interest
None.
References
- S Hema, G Reddy. A review on new analytical method development and validation by RP-HPLC. Int Res J Pharm Biosci 2017. [Google Scholar]
- A Chauhan, B Mittu, P Chauhan. Analytical method development and validation: a concise review. J Anal Bioanal Tech 2015. [Google Scholar]
- R Patil, T Deshmukh, V Patil, K Khandelwal. Review on analytical method development and validation. Res Rev J Pharm Anal 2014. [Google Scholar]
- P Ravisankar, CN Navya, D Pravallika, DN Sri. A review on stepby-step analytical method validation. IOSR J Pharm 2015. [Google Scholar]
- P Vishvas D, P Paresh U. Development and validation of simultaneous equations method for estimation of betahistine dihydrochloride and prochlorperazine maleate in tablet dosage form. Inventi Rapid: Pharm Analysis and Quality Assurance. Int J Pharm Drug Anal 2013. [Google Scholar]
- TP Devi, A Setti, S Srikanth, S Nallapeta, SC Pawar, JV Rao. Method development and validation of paracetamol drug by RP-HPLC. J Med Allied 2013. [Google Scholar]
- G Nirupa, UM Tripathi. RP-HPLC analytical method development and validation for simultaneous estimation of two drugs nitazoxanide, ofloxacin and its pharmaceutical dosage forms. Int J ChemTech Res 2012. [Google Scholar]
