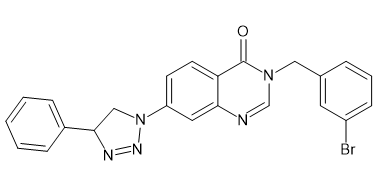
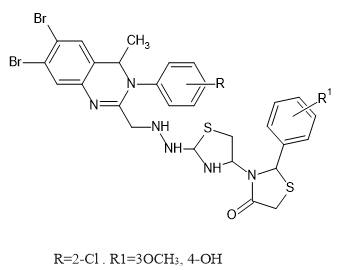
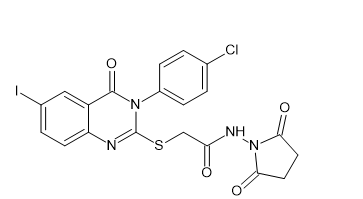
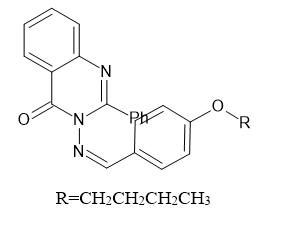
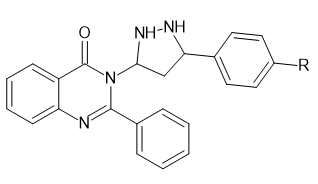
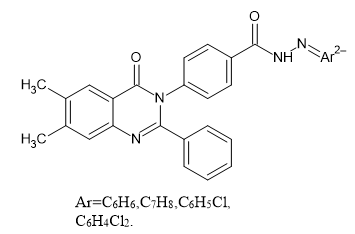
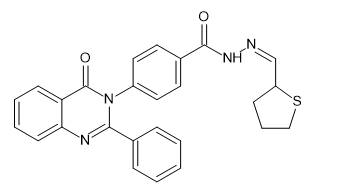
Introduction
The term ‘drug’ probably originated Arabic source and may be first mentioned in ancient German term as drug, means to a kind of powder. Nature has provided plenty of plants, fungi, insects, and reptiles which are great sources of pharmacologically active chemical substances against various co-morbidities.1
Even though nature has been providing several medication for multiple diseases or disorders that principally comprise broad spectrum class; researchers are a lot of determined towards artificial medications, because of their efficiency in slender spectrum category (specific drug for specific illness or disorder).
Most of the pharmaceutical industries synthesize medication from heterocyclic compounds. as an example, high two hundred proprietary medication of pharmaceutical industries have 75% of heterocyclic fragments in their structure. One in all the favored and interested molecule among heterocyclic compounds was a by-product of quinazoline-4(3H)-one.2
The quinazoline-4(3H)-one, previously named as benzo-1,3-diazine and its derivatives were found in more than 200 naturally occurring alkaloids. From 1903 still quinazoline formed by 3,4–dihydroquinazoline and alkaline potassium ferricyanide through oxidation.3
Most of the pharmacologically active organic cycles ideally had N atom it, either naturally or by artificial means offered prescription drugs. Between them, quinazoline and its analogues contains a superior class together with a numerous useful bioactive property over microorganism, diabetic, convulsant, tumor, malarial, hypertensive, inflammatory and enzyme conditions.4
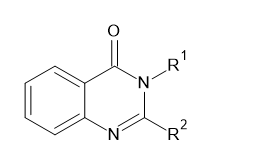
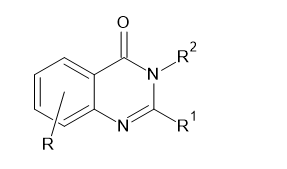
Different schemes for synthesis of Quinazolin-4(3H)-ones
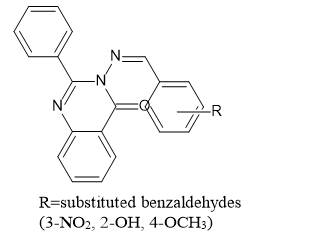
Sweta Garg et al. (2017) synthesized di-substituted quinazoline-4(3H)one(I)compound with numerous aldehydes and assessed their activity against microorganism and inflammatory conditions.5
Ramin Ghor baniVaghei et al. (2016) found that economical and one-pot synthesis of novel quinazoline-4(1H)-one(I) derivatives by following inexperienced chemistry ethics.6
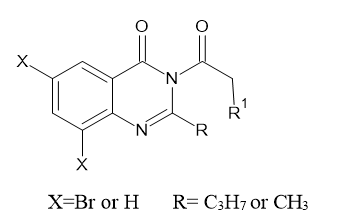
D.A. Patil et al. (2011) synthesized of bioactive two, 3-disubstituted-quinazoline-4(3H)- one(I) compounds.7
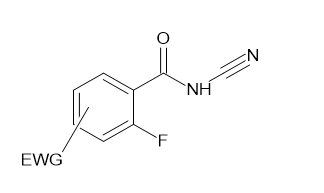
Martin J et al. (2001) synthesized 4(3H)-quinazolinones(III) from 2-Fluoro substituted benzoyl chlorides(I) with 2-amino-N-heterocycles(II) by cyclo-condensation reaction. The reaction profits suitable yields with a numerous combinations of benzoyl chloride and 2-amino-N-heterocycles.8
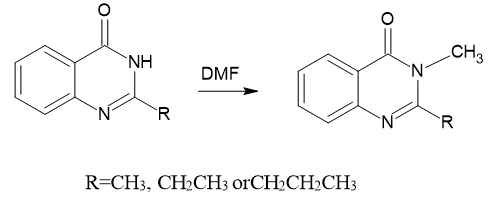
Mohammad Rafeeq et al. (2015) evaluated the potential of N, N-dimethyl formamide dimethyl acetal (DMF-DMA) as a methylating substance that was used for the reactions of 2-alkyl substituted quinazolin-4(3H)-one(I) in solvent free condition.9
Antitumor Activity
Nulgumnalli M R et al. (2009) synthesized many imidazolyl-(4-oxoquinazolin-3(4H)- yl)-acetamides(I) and screened them for them against Ehrlich Ascites Carcinoma. The compounds had moderate antitumor activity compared to reference drug.10
Subhadip Das et al. (2012) presented the evaluation of the antitumor activity of these quinazolinones(I).11
Sarah T et al. (2006) prepared a sequence of quinazoline analogs to look like methotrexate structure. The synthesized derivatives were evaluated for their capacity to reduce mammalian DHFR and for their antitumor activity. 12
Srikanth Gatadi et al. (2020) Synthesized a sequence of novel quinazolinones(I) and estimated their cytotoxic activity against a various human cancer cell line. They showed apoptosis, chromatin condensation and horseshoe shaped nuclei appearance.13
Anticonvulsant Activity
Neha Garg et al. (2009) synthesized a novel analog of 3-(4-(2-(6,8-dibromo-3- (substituted phenyl)-4-oxo-3,4 dihydroquinazolin-2-yl)methyl) hydrazinyl)thiazol-2-yl)- 2-phenylthiazolidin-4-ones(I) and a few of its derivatives showed effective anticonvulsant activity.
Adnan A. Kadi et al. synthesized a novel sequence of 2-mercapto-3-(4-chlorophenyl)-4- oxo-6-iodoquinazoline. The anticonvulsant activities of prepared derivatives screened with the pentylenetetrazole -seizure threshold test. Compound showed a noteworthy anticonvulsant action.14
Mohd Amir et al. (2013) prepared a sequence of 3-[(4-substituted-benzylidene)-amino]- 2-phenyl-3H-quinazolin-4-ones. Anticonvulsant actions were screened by the maximal electric shock and subcutaneous Pentylenetetrazole. Compound 3-[(4-butoxy- benzylidene)-amino]-2-phenyl-3H-quinazolin-4-one(I) was came out as the mainly capable anticonvulsant agent lacking any motor impairment result.15
Anjali G et al.(2011) synthesized a series of 3-[5-(4-substituted) phenyl- 1,3,4- oxadiazole-2yl]-2 phenylquinazoline-4(3H)-ones(I). They were observed with moderate action in the maximum electric shock seizures and PTZ provoked seizure models in rats.16
Antimicrobial Activity
V. K. Singh et al. (2013) synthesized Quinazolin-4(3H)-ones(I) and a few of those derivatives exhibit higher anti-bacterial activity.17
Mohamed T et al. (2010) synthesized 5-Amino-4-cyano-1H-pyrazole (I) derivatives. The synthesized derivatives showed moderate to high inhibition actions. Derivatives add in with a sugar moiety and a pyrazolyl ring in their structure showed the most antimicrobial activity.18
Conclusion
The literature survey reveals that structural modification around the fused ring, quinazoline derivatives helpful for treatment of various disease conditions. Most of the publications appeared within the literature is based on the synthesis of quinazolines using conventional methods. Thus, we can conclude that this review will definitely provide researcher a thorough understanding of the numerous biological activities which further helps in designing several range of good quinazoline derivatives.