Introduction
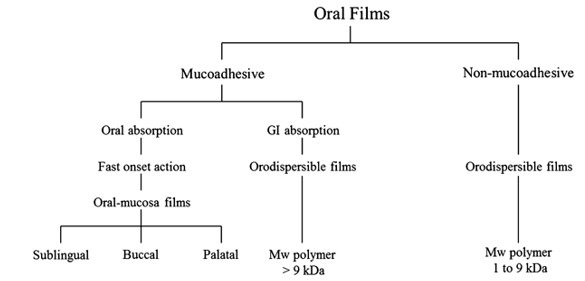
“An orally disintegrating film is a film which dissolves in a mouth is generally called as orodispersible film by European Medicine Agency and simply called as oral soluble film by Food and drug administration”.1
'Orally disintegrating film are mostly composed of complex polymeric matrices that serve as drug delivery platforms.' 2 "These polymeric matrices are made up of a variety of components to create well-designed drug delivery platforms, although the basic core of the system is usually hydrophilic polymer." Polymers were first introduced into pharmaceutical and biomedical manufacturing as significant components of formulations and packaging, and their applicability quickly spread to a wide range of applications, including the most modern drug delivery systems and devices.2
'Low molecular weight (about between 1.000 and 9.000 Da) hydrophilic polymers are generally used in nonadhesive quick dissolving flicks.' These films are designed to show a rapid decomposition in the oral cavity, be ingested, and absorbed into the GIT's systemic rotation.2
Advantages
"The huge surface area of the oral cavity causes fast break down and disintegration of the oral dose form."5, 6
Orally disintegrating films are solid unit dosage forms that provide precise dosing and stable performance.
The bioavailability of the medicine is enhanced due to absorption through the buccal cavity, and minimal doses are required, increasing patient compliance.5, 6
"Because an orally dissolving film does not require water to swallow, it is a superior choice for dysphasic people."
Limits
"This technology, like any other delivery system, has its own set of restrictions. The process of combining water-insoluble medicines with an oral thin film is still in its early stages. Components that aid in the dissolution of a medicine can be included in tablets and capsules.7 Oral thin films, on the other hand, are a more modern drug delivery technique that largely uses polymers to boost a drug's solubility. ” 'To boost the solubility of water-insoluble medications in oral thin films, scientists are investigating promising new particle engineering approaches.' "Other limitations of oral thin films include the fact that they can only carry a tiny medication load of around 10 to 20 mgs. Oral thin films are also more susceptible to humidity and temperature due to their larger surface area. Fortunately, appropriate packaging can keep oral thin films safe in a variety of situations."8
Application9, 10
Orally dissolving films are used to treat localised discomfort, allergies, sleeping problems, and CNS issues.'9, 11
Soluble films are appropriate for topical administration as analgesics or antibacterial agents in wound treatment.'9, 11
Orally disintegrating films can be used to improve the bioavailability of medications that are poorly bioavailable.9
Topical application of dissolvable films as analgesics or antibacterial agents for wound treatment is possible.'12, 10
Unpleasant medications are hidden in the taste.
Formulation consideration4, 13
All excipients used in the formulation and development of orally disintegrating film are considered safe (GRAS listed) and should be permitted for use in oral pharmaceutical dosage forms from a regulatory standpoint. Oral thin films have a surface area of 1-20cm2 (depend on dose and drug loading containing drug).
Drug
The active pharmaceutical ingredient is typically found in a film at a concentration of 1-30% w/w. Micronized API enhances the texture of the film and provides rapid dissolution and homogeneity in the fast dissolving film, making it crucial for optimal formulation.14
For the development of oral strip formulations, the desired qualities of the medicine:9
Polymer
In the creation of films, polymers play a vital role. The preparation uses hydrophilic polymers such that the film dissolves quickly in the mouth cavity and the medicine is given to the blood stream via dissolving when it comes into touch with salivary in the mucous membrane.5
Water-soluble polymeric are employed as film modifiers because they provide fast disintegration, a pleasant mouth feel, and good mechanical qualities. Polymers can be employed alone or in conjunction with others to provide desirable film qualities such as hydrophilic nature, elasticity, taste, and solubility. The rate of polymer disintegration is done by reducing the molecular mass of polymer film bases.8
The polymers utilised in the oral film have following ideal characteristics: 5
Non-toxic and non-irritant polymers should be used.
It must be non-bitter; polymers must be tasteless; it must be free of leachable contaminants; and it must be affordable and readily available.
It should not obstruct the disintegration process
It must have good hydration and spreadibility
It should have enough peel, shear, and tensile strength
It should have a long shelf life
It should not result in a serious infection in the mouth.
Plasticizers12
Emollients are important excipients for oral films. It improves film flexibility and mechanical properties (tensile strength, elongation, etc.) and reduces strip brittleness. Plasticizers significantly improve tape properties by lowering the glass transition temperature of the polymer. Plasticizers should be chosen to be compatible with drugs, polymers, and other excipients used in oral films. Plasticizers can improve flowability and increase polymer strength. Using the wrong plasticizer will cause the film to tear, tear or delaminate. Plasticizers are used in concentrations of 0-20% by weight of the dry polymer weight. Various plasticizers used in the preparation of orally dispersible films are glycerin, propylene glycol, polyethylene glycol, dimethyl, dibutyl, diethyl phthalate, tributyl, triethyl, acetyl citrate, triacetin and castor oil. 12
Sweetening agent12
Commonly, sweeteners are used to mask the bitter taste of medicines and make them palatable. Sweeteners are used singly or in combination at concentrations of 3-6% by weight. Both natural and artificial sweeteners are used in the production of oral films. Xylose, ribose, glucose, sucrose, maltose, stevioside, dextrose, fructose, liquid glucose, isomaltose are used as natural sweeteners. Fructose is sweeter than sorbitol and mannitol, so it is widely used as a sweetener. Artificial sweeteners used in oral dissolving films are saccharin sodium or calcium salts, cyclamate, acesulfame K, etc. Acesulfame K and Sucralose have 200 and 600 times more sweetening power.12
Flavoring agent
Flavouring agents are added in order to improve the acceptance by the patients. Up to 10% w/w concentration of flavours are added. Flavouring agents are selected on the basis of type of the drug incorporated. Age plays important role in the selection of the flavouring agents. Paediatric patients like chocolate and fruit flavours, but geriatric patients like orange and mint flavours. Flavouring agents are extracted from the plants fruits and flowers.eg: vanilla, orange, peppermint.
Coloring agent
Colouring agent imparts colour to the formulation. Colouring agents are selected according to the flavour. FD&C approved Colouring agents are incorporated in the oral film these are also important ingredients in order to improve the appearance of the formulation. It is used up to 1%w/w. e.g. titanium dioxide.
Saliva stimulating agents
They increase salivary secretion, allowing the oral film in the mouth to break down and dissolve more quickly. Acids used in food preparation are commonly used as saliva stimulants. These agents are used alone or in combination at 2-6% by weight of the oral strip. Citric, malic, lactic, ascorbic, and tartaric acids are salivating agents. Of these, citric acid is most preferred. Simultaneously comparing the resting saliva volume and the stimulated saliva volume under the same conditions allows the stimulation of salivary secretion to be measured.12
Method of preparations
A) Casting
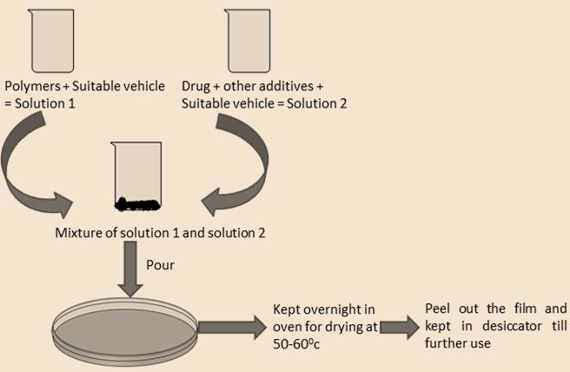
a) Solvent casting
Is the most commonly used method for the preparation of ODFs using water soluble excipients, polymers and drug which are dissolved in de-ionized water; consequently, a homogenous mixture is obtained by applying high shear forces generated by a shear processor.
The API is either suspended or dissolved in a solution of polymers, plasticizers, and any other ingredients dissolved in a volatile solvent, like water or ethanol.
In this method firstly water soluble ingredients are mixed in water to form viscous solution.
b) Semisolid casting method
When acid insoluble polymers are to be used in the preparation of films, this method is applied. In this method gel mass is casted in to films, gel mass is obtained by adding solution of film forming to a solution of acid insoluble polymer present in ammonium or sodium hydroxide this is rolled dried and then cut.
The solution is added to a solution of acid insoluble polymer. Plasticizer is added in the appropriate amount so that a gel mass is formed. The gel mass formed is then casted into the films or ribbons by using heat controlled drums. Acid insoluble polymers used to prepare films include: cellulose acetate phthalate, cellulose acetate butyrate. The thickness of the film is about 0.015-0.05 inches. Acid insoluble polymer and film forming polymer are used in the ratio of 1:4.
Extrusion
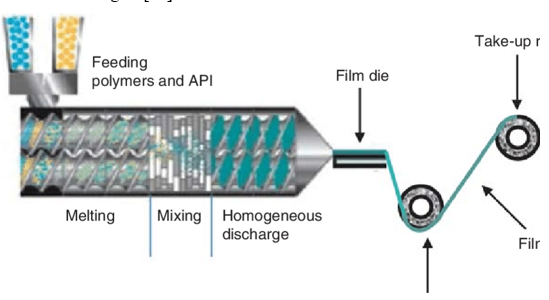
Hot Melt Extrusion
In this method, the drug is mixed with the carrier in solid form. Then dry granules are introduced. The dried granules are then fed into the extruder. To process the granules in the extruder barrel for approximately 3-4 minutes, the screw speed should be adjusted to 15 rpm. The processing temperature is 800°C and it is pressed into a cylindrical calender to create a film. Hot melt extrusion has certain advantages. 10
Solid dispersion
In the solid dispersion extrusion process, drug-immiscible ingredients are extruded and then a solid dispersion is prepared. Finally, solid dispersions are formed into films using dyes 10
Rolling method
In this method firstly solution or suspension of drug is prepared which have certain rheological consideration. Either water or mixture of water and alcohol is mainly used as solvent. Suspension or solution containing drug is rolled on the carrier. Films are dried on the rollers and cut into desirable shapes and sizes.
Evaluation of orally disintegrating film
Mechanical properties
Thickness
Dryness/tack test
Water uptake
Tensile strength
Percent elongation
Young’s modulus
Tear resistance
Folding endurance
-
Determination of film weight
Thickness: A thickness of film should be measured with the help of micrometer screw gauge or calibrated digital vernier callipers. Film should be measured at five points i.e. from the centre and from all the four corners and then mean thickness is calculated. It is necessary to determine the uniformity of thickness as it is directly related to accuracy of dose in the film.
Dryness/ Tack test: Dryness is the property to measure the solvent or water content present in the film whereas tack is the tenacity with which the film adheres to any piece of paper which is pressed into contact with the strip. Eight stages of film drying process have been recognized i.e. set-to-touch, dust free, tack-free, dry-to-touch, dry-hard, dry-through; dry-to-recoat & dry print free. Now instruments are also available to study.
Determination of water uptake: The weight of a 2 cm x 2 cm film was determined (W) The 1 film was soaked in water and allowed to swell for 1 hour and the final weight (W) was determined after patting 2 gently with a filter paper. The percentage water uptake was determined from the equation:% water uptake = W2 — W1 × 100
Tensile strength: This is the maximum stress applied at one point on the film that causes the tab sample to fail. It is calculated by dividing the applied breaking load by the cross-sectional area of the strip as given by the following formula: Tensile Strength = Load at Break * 100 / Strip Thickness * Strip Width12
Percent Elongation: A film sample stretches when a load is applied. This is called elongation. Elongation is basically the deformation of the film divided by the original dimensions of the sample. In general, film elongation increases with increasing plasticizer content.12
Young's Modulus: Young's modulus or elastic modulus is a measure of the stiffness of a film. It is expressed as the ratio of applied stress and strain in the region of elastic deformation as follows: Young's Modulus = Pitch x 100/Film Thickness x Crosshead Speed Hard, brittle films exhibit high tensile strength and high Young's modulus at low elongations.12
Tear Resistance: The maximum stress or force required to burst the film (usually found near the onset of burst) is recorded as the Burst Strength value in Newton’s (or Pound Force).12
Determination of fold endurance: The 2 cm x 2 cm film was repeatedly folded at the same place until the film cracked. The number of times the film7 was folded was denoted as the fold endurance value. The experiment was performed in triplicates and average value was determined.
Determination of film weight: Six films of 2 cm x 2cm from each batch were weighed on an analytical balance (Mettler Toledo, USA) and the average weight of the films was determined.
Organoleptic properties
This is an essential step in most oral formulations due to their long residence time in the oral cavity. Products should possess the desired characteristics of sweetness and flavor that are acceptable to a large portion of the population. A specially controlled human taste panel is used to assess the psychophysical ratings of the products. For this purpose, in vitro methods using taste sensors, specially designed devices, and drug release by modified pharmacopoeial methods are used. Experiments using electronic tongue measurements to distinguish between sweetness levels in taste-masked formulations have also been reported.12
Swelling test
A simulated salivary solution is used to conduct studies of swelling properties. First, weigh all foil samples and place them on a pre-weighed stainless steel wire grid. Place 15 ml of saliva solution in a plastic container and immerse the foil sample containing the screen in it. The weight of the film was observed to increase until a constant weight was observed.12
The degree of swelling was calculated using parameters:
α = wt-wo/wo
Wt= weight of film at time t
WO= weight of film at time zero
Surface ph. test
The surface pH of film should be 7 or close to neutral.
In method to determine the surface pH, the films are placed on the 1.5%w/v agar gel and then the pH paper are placed on the film, change in colour of pH paper gives surface pH of the film.
Contact angle
Contact angles are measured at room temperature using a goniometer (AB Lorentz and Wettre, Germany). Take a dry film and place a drop of distilled water on the surface of the dry film. Images of water droplets were taken within 10 seconds after deposition using a digital camera. Contact angles were measured on both sides of the droplet and averaged.15
Transparency
Film transparency can be determined using a simple UV spectrophotometer. Cut the film into rectangles and place them in the spectrophotometer cell. Measure the transparency of the film at 600 nm.15
The transparency of the film was calculated as follows:
Transparency= (log T600)/b = ―є
Where, T600= transmittance at 600nm
b= film thickness (mm)
C= concentration
Content uniformity
This is determined by standard test procedures described for specific APIs in standard pharmacopeias. Indicates the amount of medicine in each strip.15
Limit of content uniformity is 85-115%.
Example. Content uniformity test of losartan
Drug content of all formulations was determined by UV spectrophotometry. For this purpose, films measuring 2×2 cm 2 were cut and dissolved in 100 ml of pH 6.8 phosphate buffer. The solution was filtered and the absorbance recorded at 206 nm. The drug content was calculated from the drug calibration curve. All readings were obtained in triplicate.16
Disintegration test
The disintegration time limit is 90sec or less. Although no official guidelines is available for oral strips. Pharmacopoeia disintegrating test apparatus may be used for the study. Typical disintegration time for oral strip is 5- 30sec.
It was observed that as the concentration of polymer increased, the thickness of the film increased, thereby increasing the time required for the film to disintegrate. The rapid degradation of MDF with increasing plasticizer concentration was due to rapid water uptake by the hydrophilic plasticizer, followed by swelling and immediate hydrogen bond scission.16
In vitro dissolution test
Dissolution testing can be performed using standard basket or paddle apparatus as described in pharmacopoeias. The dissolution medium is selected essentially according to sink conditions and the highest drug dose. Dissolution testing can often be difficult when using a paddling device as the strip tends to float in the eluate.17
Discussion
This review concludes that oral dissolving films are the most acceptable and precise oral dosage forms that bypass first-pass metabolism and exhibit more pharmacological activity. Melt-in-the-mouth films are gaining importance in the pharmaceutical sector. They offer many advantages over other dosage forms beyond simple manufacturing and evaluation techniques. Oral films can replace over-the-counter (OTC), generic, and branded drugs on the market due to their low cost and consumer preference. This technology is an excellent product life cycle management tool that extends the patent life of existing products. This review is an attempt to combine the available knowledge on melt-in-the mouth films. Much research work is underway and will be initiated on melt-in your-mouth films in the near future.
Conclusion
This drug delivery system is innovative for all patient groups that have swallowing problems like paediatric, geriatric patients. It gives many advantages over other dosage form like improving drug bioavability and faster onset of action. Because drug absorption takes place in oral cavity drug that degrade by gastric acid and inactive by first pass metabolism are also incorporated in orally disintegrating film.
This dosage form has rapid termination of therapy in case of wrong drug delivery, overdose, and incompatibility and in allergic condition. In case of emergence due its onset and quick action it is most suitable dosage form.
Therefore, it can be concluded that the fast dissolving orally disintegrating film with excellent patient compliance and many advantages have broad futuristic opportunities.