Introduction
Now-a-days Type-2 Diabetes Mellitus is becoming increasingly prevalent, primarily due to obesity and physical inactivity among the people. This leads to be associated with an increased health care resource burden, which includes especially, cost of anti-diabetic agents, hospital inpatient care and treatment complications of diabetes which might be microvascular or macrovascular complications.1 It is an interesting fact that should be known that kidney plays a major role in the regulation of blood glucose levels in body i.e., in individuals, the kidneys filter a large volume of glucose and actively reabsorb virtually all of it. Glucose reabsorption is necessary to retain calories, but becomes counterproductive in type 2 diabetes mellitus. Thus because of this, in hyperglycemic patients, a greater amount of glucose is filtered and reabsorbed by the kidneys, which causes sustained hyperglycemia. In prolongation it leads to sustained hyperglycemia contributing to insulin resistance and leading to dysfunction in the beta cells of the pancreas further undermining control of the disease2. Thus primary treatment of goal of management of diabetes management is to reduce the glycemic levels, lowering glycated hemoglobin (HbA1c) levels to below or around 7%.1 The efficacy of common anti-diabetic drugs (including metformin, sulfonylureas, nonsulfonylureasecretagogues, alpha glucosidase inhibitors, thiazolidinediones, glucagon- like peptide-1 analog and dipeptidyl peptidase-4 inhibitors) are insulin dependent.2, 3 Current Oral Anti-Diabetic therapy leads to events like weight gain, hypoglycemia.2 Thus because of these problems several new molecule syntheses are taking place for the management of diabetes avoiding the insulin resistance development.
Dapagliflozin (INN/USAN trade name Farxiga in the US and Forxiga in the EU) is a drug which was developed by Bristol-Myers Squibb in partnership with AstraZeneca.
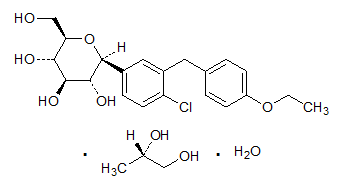
Chemical Structure of Dapagliflozin Propanediol Monohydrate
Molecular Formula: C21H25ClO6
Molecular weight of Dapagliflozin: 408 873 g/mol
Molecular weight of Dapagliflozin Propanediol Monohydrate: 502 98 g/mol
Dapagliflozin a new agent of oral anti-diabetic group belongs to the class selective sodium glucose co-transporter-2 (SGLT-2) inhibitor, an agent that has been recently introduced for the treatment of Diabetes Mellitus-2. It is the drug that reduces the glucose level by inhibiting SGLT-2, dapagliflozin reduces renal glucose reabsorption, causing the increased urinary excretion with excess glucose thus causes the reduced glucose concentrations.4 The ATC Code of Dapagliflozin is A10BX09 and Dapagliflozin + Metformin is A10BD15.5 Also Dapagliflozin is not the first member of SGLT-2 inhibitor and the first member is Canagliflazolin and got approved on March 29, 2013.
To get approved by US-FDA, the New Drug Application 202293 was filed for Dapagliflozin on December 28, 2010 and the molecule got approved on January 2014.
Drug properties
An oral hypoglycemic drug under the class of sodium-glucose transport proteins (SGLT2) film coated tablet contains dapagliflozin as active ingredient and microcrystalline cellulose, anhydrous lactose, crospovidone, silicon dioxide, magnesium stearate as inactive ingredients. Also, the film coat has the inactive ingredients: polyvinyl alcohol, titanium dioxide, polyethylene glycol, talc and yellow iron oxide and approved as a monotherapy.6 These tablets are to be consumed once daily in the morning and with or without food. The active ingredient was synthesized via C-arylation of 2, 3, 4, 6-tetra-O-trimethylsilyl-l-d-glucolactone with a benzophenone derivative. Chemically it is (2S, 3R, 4R, 5S, 6R)-2-[4-chloro-3-(4-ethoxybenzyl) phenyl]-6-(hydroxymethyl) tetrahydro-2H pyran-, 4, 5-triol).7 The storage precaution reveals that, the drug should be stored at a controlled room temperature between 20 to 25 degrees C (68- and 77-degrees F); excursions permitted between 15 to 30 degrees C (59- and 86-degrees F).8
Mechanism of Action
Dapagliflozin is a potent, highly selective reversible, orally active competitive sodium glucose co-transporter-2 (SGLT2) inhibitor with potency at nanomolar stage that effect in the maintenance of glycemic control in patients with type 2 diabetes mellitus by reducing renal glucose reabsorption leading to urinary glucose excretion (glucuresis).2, 6, 9
SGLT-2 is selectively expressed in the kidney with no expression detected in more than 70 other tissues including liver, skeletal muscle, adipose tissue, breast, bladder, and brain. SGLT-2 is the predominant transporter that causes the reabsorption of glucose from the glomerular filtrate back into the circulation. This does not affect the insulin secretion but continues to inhibit the reabsorption of filtered glucose.6 Even by this inhibition, it causes maintenance of both fasting and post-prandial plasma glucose levels, thus it results in the consumption of fat as an energy source.6, 10 But Glomerular Filtration Rate (GFR) and blood glucose concentration are the factors on which the amount of glucose removed by the kidney through this mechanism is depended. Thus, in healthy subjects with normal glucose, dapagliflozin has a low propensity to cause hypoglycemia. Dapagliflozin acts independently of insulin secretion and insulin action. Over time, improvement in beta cell function (HOMA-2) has been observed in clinical studies with dapagliflozin.6
Indications
Dapagliflozin is used as an adjunct to diet to improve glycemic control in adults with type-2 diabetes mellitus. But it should not be used for patients with type 1 diabetes mellitus or for the treatment of diabetic ketoacidosis.6
Pharmacokinetic properties
1. Absorption: Following the oral administration of drug, maximum plasma concentration (Cmax) is usually attained within 2 hours under fasting state. The Cmax and AUC values increase dose proportionally with increase in dapagliflozin dose in the therapeutic dose range. 78% of absolute bioavailability is attained by 10mg of dapagliflozin drug administered. Also, with co-administration of high fat meal, it causes decreased in its Cmax by upto 50% and prolongs Tmax by approximately 1 hour, but does not alter AUC as compared with the fasted state. But they are not clinically meaningful and dapagliflozin can be administered with or without food.8
2. Distribution: It is approximately 91% protein bound. Protein binding is not altered in patients with renal or hepatic impairment.8
3. Metabolism: The metabolism pathway of Dapagliflozinis mainly mediated by UGT1A9; CYP mediated metabolism is a major clearance pathway in human. Drug is extensively metabolized, primarily to yield dapagliflozin 3-O- glucuronide, which is an inactive metabolite8. Dapagliflozin 3-O- glucuronide (BMS 801576). The primary metabolite accounts for 61% of the dapagliflozin dose.4
4. Excretion: Dapagliflozin and related metabolites are primarily eliminated by the renal pathway. Following a single dose 50mg dose of [14C]-dapagliflozin, 75% and 21% total radioactivity is excreted in urine and feces, respectively. In urine less than 2% of the dose is excreted as the parent drug. In feces approximately 15% of the drug is excreted as parent drug. The mean plasma terminal half life (t1/2) for dapagliflozin is approximately 12.9 hours following a single oral dose.8
Pharmacodynamic properties
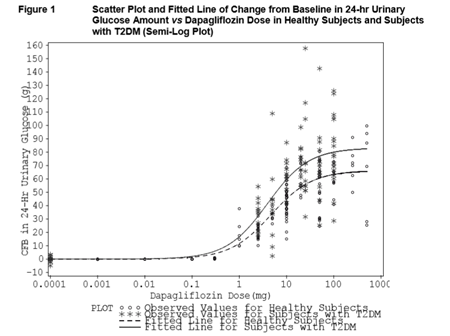
Increases in the amount of glucose excreted in the urine were observed in healthy subjects and in patients with type 2 diabetes mellitus following the administration of dapagliflozin (Figure 1). Approximately 70 g of glucose was excreted in the urine per day (corresponding to 280 kcal/ day) at a Dapagliflozin dose of 10 mg/day in patients with type 2 diabetes mellitus for 12 weeks. This glucose elimination rate approached the maximum glucose excretion observed at 20 mg/day dose of dapagliflozin. Evidence of sustained glucose excretion was seen in patients with type 2 diabetes mellitus given dapagliflozin 10mg/day for up to 2 years. This urinary glucose excretion with dapagliflozin also results in osmotic diuresis and increases in urinary volume. Urinary volume increases in patients with type 2 diabetes mellitus treated with Dapagliflozin 10 mg were sustained at 12 weeks and amounted to approximately 375 mL/day. The increase in urinary volume was associated with a small and transient increase in urinary sodium excretion that was not associated with changes in serum sodium concentrations.
Urinary uric acid excretion was also increased transiently (for 3-7 days) and accompanied by a reduction in serum uric acid concentration. At 24 weeks, reductions in serum uric acid concentrations ranged from 18.3 to 48.3 μmol/L.6
Precautions
Hypotension: Dapagliflozinleads to intravascular volume contraction. Symptomatic hypotension may occur after initiating the drug especially in renal impaired patients (eGFR less than 60mL/min/1.73m2), elderly patients or patients who are on loop diuretics. Thus, before initiating this drug in patients with these characteristics, volume status should be assessed and corrected.
Impairment in Renal Function: Dapagliflozin elevates the Serum Creatinine and causes reduction in the eGFR. Especially elderly patients and renal impaired patients are more susceptible to these changes. Also, adverse reactions can be seen in patient related to renal function. Thus, renal function should be evaluated prior to initiation of drug therapy and monitored periodically thereafter.
Hypoglycemia with Contaminant Use with Insulin and Insulin Secretagogues: Insulin and Insulin Secretagogues are known to cause Hypoglycemia.Dapagliflozin can increase the risk of Hypoglycemia when combined with Insulin and Insulin Secretagogues. Therefore, a lower dose of insulin or insulin secretagogues may be required to minimize the risk of hypoglycemia when these agents are used in combination with Dapagliflozin.
Increases in Low Density Lipoprotein Cholesterol (LDL-C): Increases in LDL-C can occur with Dapagliflozin. Monitor LDL-C and treat per standard of care after initiating the Dapagliflozin.
Bladder Cancer: Across 22 clinical studies, newly diagnosed cases of bladder cancer were reported in 10/6045 patients (0.17%) treated with Dapagliflozin and 1/3512 patients (0.03%) treated with placebo/comparator. After excluding patients in whom exposure to study drug was less than one year at the time of diagnosis of bladder cancer, there were 4 cases of Dapagliflozin and no cases with plabebo/comparator.Also, the available data are insufficient to determine whether Dapagliflozin influences pre-existing bladder tumors. So, Dapagliflozin should not be used in patients with active bladder tumors. In patients with prior history of bladder cancer, the benefit of glycemic control versus risks of cancer reoccurrence with Dapagliflozin should be considered.8
Drug interactions
There were no clinically meaningful drug-drug interactions observed with several concomitant medications used by T2DM patients, like metformin, sitagliptin, digoxin, simvastatin, and warfarin. No drug-drug interactions were observed with drugs that may affect the metabolic pathway of Dapagliflozin (e.g., mefenamic acid and rifampin). Also, they demonstrated that dapagliflozin has little potential either to affect metabolism or to have its metabolism meaningfully affected by co administration of other drugs. The mean exposure to dapagliflozin in subjects with moderate and severe hepatic impairment was 36 % and 67% higher, respectively, than that of healthy subjects. 4
Pregnancy and Lactation Category
Pregnancy Category: There are no adequate and well-controlled studies of Dapagliflozin in pregnant women. Based on the results of reproductive and developmental toxicity studies in animals, Dapagliflozin can affect renal development and maturation during the second and third trimesters of human pregnancy. Therefore, Dapagliflozin must not be used during the second and third trimesters of pregnancy. Also, after detecting that patient is pregnant, the drug should be discontinued. 8, 9 Thus the drug is included in Pregnancy Category C8 but due to absence of data from the use in pregnancy it is also included in Pregnancy Category D 9 according to a literature.
Lactation Category: It is not known either Dapagliflozin or metabolites are excreted in human milk. 6 Dapagliflozin is excreted in rat milk reaching levels 0.49 times that found in maternal plasma. 8
Dosing information
Currently Dapagliflozin is available in 5mg and 10mg film coated tablets. Prior to initiating therapy, it is necessary to determine the renal function and volume status. Also correct the volume depletion prior to initiating therapy. The recommended starting dose of Dapagliflozin is 5mg once daily, taken in the morning, with or without food. In patients tolerating Dapagliflozin 5mg once daily who require additional glycemic control, the dose can be increased to 10mg once daily. 8
Dosage in renal failure
1. Dosage adjustment is not required in patients with mild renal impairment (eGFR of 60mL/min/1.73m2 or greater).
2. But dapagliflozin should not be administered in patients with an eGFR less than 60mL/min/1.73m2. But the drug should be discontinued if the eGFR falls persistently below 60mL/min/1.73m2 during the treatment. 8
Dosage in Other Disease States
When dapagliflozin is used in combination with Insulin or Insulin secretagogues, there may be an increased risk of hypoglycemia. A lower dose of insulin or insulin secretagogue may be required to minimize this risk. 8
1. Over dose: In healthy subjects at single doses of oral drug administration upto 500mg (50 times of MRHD), has shown that to be safeand well-tolerated. These subjects had detectable glucose in the urine for a dose-related period (at least 5 days for the 500 mg dose), with no reports of dehydration, hypotension, or electrolyte imbalance, and with no clinically meaningful effect on QTc interval. The incidence of hypoglycemia was like placebo. In clinical studies where once-daily doses of up to 100 mg (10 times the MRHD) were administered for 2 weeks in healthy subjects and type 2 diabetes patients, the incidence of hypoglycemia was slightly higher than placebo and was not dose-related. Rates of adverse events including dehydration or hypotension were like placebo, and there were no clinically meaningful dose-related changes in laboratory parameters including serum electrolytes and biomarkers of renal function.
2. In the event of an overdose, appropriate supportive treatment should be initiated as dictated by the patient’s clinical status. The removal of dapagliflozin by haemodialysis has not been studied. 6
3. Monotherapy and Add-on Combination Therapy: The recommended dose of Dapagliflozinis 10 mg once daily as monotherapy or as add-on to combination therapy with metformin, a sulfonylurea, or insulin (alone or with one or both of metformin or a sulfonylurea [SU]). 6
4. Initial Combination Therapy: The recommended starting doses of Dapagliflozin and metformin when used as initial combination therapy are 10 mg Dapagliflozin plus 500 mg metformin once daily. Patients with inadequate glycemic control on this starting dose should have their metformin dose increased according to approved metformin Product Information. 6
Monitoring parameters
Therapeutic parameters
Periodically it is important to assess glycemic control for the purpose of determining its effectiveness.11
Therapeutic goals for older adults with diabetes11
Table 2
Treatment goals may be considered based on health status and life expectancy11
Toxicity parameters
Perform renal function assessment prior to starting dapagliflozinpropanediol and periodically thereafter.
Assess the LDL cholesterol after initiating treatment.
Assess volume status before starting treatment in patients with renal impairment (estimated GFR less than 60 mL/min/1.73 m2), who are elderly, or on loop-diuretics and monitor for signs and symptoms of hypotension following initiation of therapy.
Monitor patients for genital mycotic infections during therapy, especially in patients with a history of such infections. 12
Adverse Events
Cardiovascular effects
Volume depletion had caused dehydration, hypovolemia, hypotension, or orthostatic hypotension and has been reported in 0.6% and 0.8% of patients treated with dapagliflozinpropanediol 5 mg (n=1145) and 10 mg (n=1193), respectively.
Endocrine/Metabolic effects
Dapagliflozinpropanediol 5 mg (n=1145) and 10 mg (n=1193), reported Dyslipidemia in 2.1% and 2.5% of patientsrespectively.
Marked hyperphosphatemia (serum phosphorus levels of 5.6 mg/dL or higher for patients ages 17 to 65 years or greater than or equal to 5.1 mg/dL or higher for patients ages 66 years or older) was reported in 24thweek in 1.7% of patients treated with dapagliflozinpropanediol 10 mg (n=2360).
Minor episodes of hypoglycemia (symptomatic episodes with a capillary or plasma glucose value less than 63 mg/dL or an asymptomatic episode with a capillary or plasma glucose value less than 63 mg/dL that does not qualify as a major episode) were reported in 1.5% and 0.7% of patients treated with dapagliflozinpropanediol 5 mg (n=137) and 10 mg (n=135), respectively.
Hematologic effects
Hematocrit values greater than 55% by week 24 were reported in 1.3% of patients treated with dapagliflozinpropanediol 10 mg (n=2360).
Immunologic effects
Severe hypersensitivity reactions, including anaphylaxis, severe cutaneous reactions, and angioedema, were reported in 0.3% of patients.
Renal effects
Discomfort with urination was reported in 1.6% and 2.1% of patients treated with dapagliflozinpropanediol 5 mg (n=1145) and 10 mg (n=1193), respectively.
Increased urination, including pollakiuria, polyuria, and increased urine output, was reported in 2.9% and 3.8% of patients treated with dapagliflozinpropanediol 5 mg (n=1145) and 10 mg (n=1193), respectively.
Newly diagnosed cases of bladder cancer were reported in 0.17% (10 of 6045) of patients treated with dapagliflozinpropanediol.
Urinary tract infectious disease
Urinary tract infections, including cystitis, Escherichia urinary tract infection, genitourinary tract infection, pyelonephritis, trigonitis, urethritis, kidney infection, and prostatitis were reported in 5.7% and 4.3% of patients treated with dapagliflozinpropanediol 5 mg (n=1145) and 10 mg (n=1193), respectively.
Reproductive effects
Female genital mycotic infections, including vulvovaginalmycotic infection, vaginal infection, vulvovaginal candidiasis, vulvovaginitis, genital infection, genital candidiasis, fungal genital infection, vulvitis, genitourinary tract infection, vulval abscess, and bacterial vaginitis, were reported in 8.4% and 6.9% of female patients treated with dapagliflozinpropanediol 5 mg (n=581) and 10 mg (n=598), respectively.
Genital mycotic infections were reported in 5.7% and 4.8% of patients treated with dapagliflozinpropanediol 5 mg (n=1145) and 10 mg (n=1193), respectively.
Male genital mycotic infections, including balanitis, fungal genital infection, balanitis candida, genital candidiasis, penile infection, balanoposthitis, infective balanoposthitis, genital infection, and posthitis were reported in 2.8% and 2.7% of male patients treated with dapagliflozinpropanediol 5 mg (n=564) and 10 mg (n=595), respectively.
Respiratory effects
Nasopharyngitis was reported in 6.6% and 6.3% of patients treated with dapagliflozinpropanediol 5 mg (n=1145) and 10 mg (n=1193), respectively.
Others
Influenza was reported in 2.7% and 2.3% of patients treated with dapagliflozinpropanediol 5 mg (n=1145) and 10 mg (n=1193), respectively.12
Patient Information
Advisethe patients to take Dapagliflozin as prescribed. If a dose is missed, advise patients to take it as soon as it is remembered unless it is almost time for the next dose, in which case patients should skip the missed dose and take the medicine at the next regularly scheduled time. Advise patients not to take two doses of same drug at a time.
Educate the patients about the adherence to diet, regular physical activity, periodic blood glucose monitoring and HbA1c testing, recognition and management of hypoglycemia and hyperglycemia and assessment of diabetes complications.
Advice patients to seek medical advice promptly during periods of stress such as fever, trauma, infection, or surgery, as medication requirements may change.
Educate the patients/ care takers about most common adverse reactions associated with the Dapagliflozinlike genital mycotic infections, nasopharyngitis and urinary tract infections.
Instruct the patient to immediately inform her health care provider if she is pregnant or plans to become pregnant.
6. Instruct patients to immediately inform her health care provider if she is breastfeeding or plans to breastfeed. 8
Conclusion
Due to various risk factors, co-morbid conditions and presently existing drugs may not be able to reach the glycemic target, in which the new molecule Dapagliflozin may be able to maintain the glycemic target and as it is independent of insulin secretion from pancreas and its mechanism of action, it doesn’t cause the resistance which is an advantage in such conditions; but should monitor the volume in the body which causes the adverse effects. Also, post-marketing studies should be performed extensively which helps to determine the pregnancy status and incidence of adverse events in huge population which helps to determine the safety profile of the drug in extensive path.