Introduction
Cefetamet pivoxil is an oral third generation cephalosporin class of antibiotic which is mainly used in the treatment and management of both upper and lower community acquired respiratory tract infection.1 It has an excellent antimicrobial activity against the major respiratory pathogens such as Streptococcus pneumonia, Haemophilusinfluenzae, Moraxella catarrhalis, Neisseriegonorrhoea and Enterobacteriaceae.2 Cefetamet pivoxil is used in infection caused by susceptible micro organism found in otitis media, sinusitis, pharyngo tonsillitis, acute exacerbation of chronic bronchitis, tracheobronchitis of bacterial origin pneumonia, complicated and uncomplicated UTI, acute gonococcalurethretis. Many branded and generic formulations are available in market Altamet, Recocef, Bacirom O.
Literature survey has been done on the analytical methods for analysis of Cefetamet pivoxil. The reviewed methods include analysis of Cefetamet pivoxil hydrochloride in drug substance and powder for oral suspension, spectrophotometric estimation of Cefetamet pivoxil in pharmaceutical formulation and reported few chromatographic methods in bulk, powders and other pharmaceutical dosage forms.3, 4, 5 In the present analytical research, an attempt has been made to develop and validate suitable, simple, precise, robust, rugged and accurate chromatographic RP-HPLC method for estimation of Cefetamet pivoxil in bulk and tables.6, 7, 8, 9
Materials and Methods
Drug samples
Cefetamet pivoxil and tablets formulation were procured from Alembic Limited, Vadodara.sa
Chemical and reagents
All the reagents and solvents used are pure and analytical grade and obtained from store house of KLE College of Pharmacy, Belagavi. Analytical grade acetonitrile was obtained from Merck Pvt Ltd Mumbai, HPLC gradewater was obtained from RFCL Ltd New Delhi).
Instruments and apparatus
Lachrom HPLC system consisting of Merck HITACHI pump L-7100, Column oven L-7350 connected with UV detector L-7400 was used for analysis.
Methodology
The separation of analyte in HPLC system was carried on the basis of reverse phase isocratic chromatographic mode. Solubility of Cefetamet was checked in the various polar and non-polar solvent and also through the literature search and solvents was selected for the preparation of mobile phase.
Preparation of mobile phase
Solvent system composed of acetonitrile and water in the ratio 80:20 v/v was prepared, filtered through membrane nylon filters of size 4.5 μ and sonicated for 15 minutes and used for the chromatographic separation.
Selection of wavelength of detection
Solution containing analyte in the mobile phase was scanned in the UV-Spectrophotometer between 400-200 nm and UV spectrum was obtained. Analyte showed maximum absorbance at 251 nm and hence it was used as detection wavelength in HPLC system.
Preparation of standard stock and working standard solutions
10 mg of standard drug was taken in 50 ml standard volumetric flask and dissolved in the mobile phase and further diluted to 50 ml mark with mobile phase. The above stock solution was further diluted with mobile phase to get concentration ranging from 10µg/ml to 50µg/ml.
Chromatography and qualitative analysis of Cefetamet pivoxil
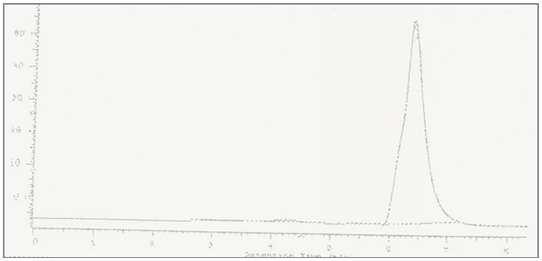
Working standard solution containing10µg/ml of Cefetamet pivoxil was injected into chromatograph composed of HYPERSIL C-18 column as stationary phase and separation of analyte was carried out using mobile phase composed of acetonitrile: water (80:20 v/v). The flow rate was with UV detection of 251 nm. The temperature of the column was ambient with average pressure was 55 psi. Chromatogram obtained was presented in Figure 2 with retention time of. Hence retention time was used as one of the qualitative parameter of Cefetamet pivoxil as it shows selective separation at minute in selected stationary and mobile phase.
Extraction of cefetamet pivoxil and sample preparation
Twenty tablets were weighed accurately and finely powdered. The powder equivalent to 10 mg of Cefetamet pivoxil was taken in a 100 ml volumetric flask, mobile phase was added and kept in an ultrasonic bath for 10 min, and further diluted to the mark and filtered through membrane filter (nylon filters) of size 4.5 μ and used for analysis. The above sample solution was further diluted with mobile phase to get concentration ranging from 10µg/ml to 20 mg/ml.
Validation of developed HPLC method
Method was validated using performing analytical parameters such as specificity, selectivity, linearity, range, precision, ruggedness, robustness, detection limit, quantification limit and drug recovery.10, 11
Specificity and selectivity
Solution containing analyte and mobile phase was injected into chromatograph and chromatograms were obtained.
Linearity and range
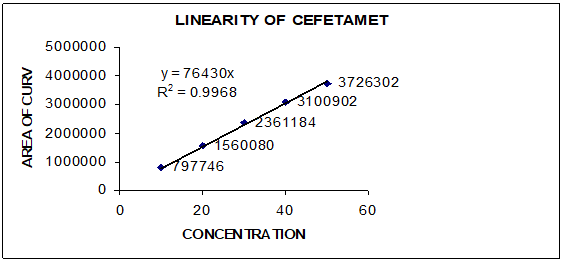
Working standard solutions containing 10µg/ml, 20µg/ml, 30µg/ml, 40µg/ml and 50µg/ml of Cefetamet pivoxil was injected into chromatograph in triplicate chromatograms was obtained and integrated. Area of each injection was taken and calibration curve was plotted using concentration vs area of analyte obtained.
Precision
Method precision was performed by injecting the solutions containing Cefetamet pivoxil in six replicates on same day at different time intervals and on different days. Chromatograms were obtained and % RSD for area was calculated.
Robustness and ruggedness
Robustness was studied by changing the ratios of solvents used in mobile phase were as ruggedness was performed by different analyst, injecting six replicates of 10 µg/ml solution into chromatograph. Area was obtained from chromatograms and % RSD was calculated.
Results and Discussion
Development
RP-HPLC estimation was based on the reverse phase isocratic chromatographic mode. Separation of analyte was carried out by using C-18 column as stationary phase. Mobile phase composed of acetonitrile: water (80:20 v/v) was used at flow rate of 0.5mL/min. analysis was performed at ambient temperature. Retention time of Cefetamet pivoxil was found to be min. (Figure 2) optimized chromatographic parameters are presented in Table 1.
Validation
The method was found to be specific and selective as the chromatogram of blank sample showed no peak and interference of any area at the retention time of Cefetamet pivoxil in chromatograms obtained. Cefetamet pivoxil showed linear response between the concentrations of 10 – 50 µg/ml with regression coefficient of 0.9991. Standard calibration curve of Cefetamet pivoxil was showed in Figure 2 and data of linearity and range was presented in Table 2. Method was found to be precise as the % RSD calculated for retention time and peak area in six replicates of injections in chromatograms was found to be less than 2% (Table 3) also the method was found to be rugged with % RSD of less than 2% for retention time and area obtained in chromatograms (Table 4). Detection limit and quantification limit of Cefetamet pivoxil was found to be 2.66µg/ml and 8.07µg/ml. Method was found to be accurate as the % recovery obtained at three different levels was found to be between 99 to 100% (Table 5). The assay results was found to be99.60%. The results of validation parameters were found to be well within the acceptance and presented in Table 6.
Table 2
linearity and range data of Cefetamet pivoxil
Table 3
Precision data of Cefetamet pivoxil
Table 4
Ruggedness data of Cefetamet pivoxil
|
Replicates |
Retention time |
Peak area |
|
1 |
6.36 |
793026 |
|
2 |
6.36 |
798842 |
|
3 |
6.35 |
798642 |
|
4 |
6.35 |
800721 |
|
5 |
6.35 |
795420 |
|
6 |
6.36 |
799021 |
|
%RSD |
0.086% |
0.355% |
Table 5
Recovery data of Cefetamet pivoxil
Table 6
Method validation report of Cefetamet pivoxil
Conclusion
RP-HPLC analytical method was developed for the estimation of Cefetamet pivoxil in bulk powder and validated as per the ICH guidelines. Developed and validated method was applied for the content estimation of analyte in its marketed formulation. All the results obtained was found to be within the acceptance limit of the guidelines also developed method was found to be simple, selective, specific, precise, accurate and rugged and can be used for the routine quality control of drug in bulk and dosage forms.