Introduction
Nature continually acts as an excellent supply rescue for creature by providing totally different remedies from its plants, animals, and different sources to cure all ailments of mankind. The Several species of plants containing substances of healthful price area unit comprised from the kingdom Plantae, that area unit however to be explored.1
The genus Launaea sarmentosa (Wild), domestically called Pathari. It is a prostate, creeping, fleshy, perennial herb that is found on sandy beaches and is distributed across Mozambique, South Africa, Madagascar, Seychelles, Reunion, Mauritius, and India at an altitude vary of zero to fifteen m.2 The leaves, stems, barks, flowers and underground elements of healthful plants are most often used for traditional medicines. Disease-treating formulations and treatment were supported healthful plants from ancient time, that practiced by the the normal physicians.3 Launaea sarmentosa is additionally according to possess tonic, soporific, diuretic, and aperient properties employed as a substitute for genus Taraxacum (Taraxacumofficinale).4 These studies enclosed of pharmacognostical analysis. Phytochemicals like Tannins, Flavonoids, Terpanoids, Steroids, Alkaloids, and Glycosides showed antimicrobial, anthelmintic, antidiarrheal activity.5, 2
Materials and Methods
Plant material
Collection and drying
Leaves of Launaea Sarmentosa were collected of local area form Sakur. Cleaned and dried at room temperature in shade and away from direct sunlight. The dried leaves were coarsely powdered in grinder. Large difference in partical size of crude drug result in long extraction time as the course partical increase the extraction time and fine powdered material was sieved through 60-120 mesh to remove fines and large particles and the powder was subjected for further study.
Macroscopic Study6
The macroscopic study is morphological description of the plant parts which are seen by eyes or magnifying lens.
Powder microscopic feature7
The microscopic study of powder is done by using the Motic electronic microscope In that the phloroglucinol and conc. HCL is add as (1:1) with powder and pink colour is observed for elongated cells. Powder and acetic acid shows the insoluble calcium oxalate crystal. Different observations were done for the powder characteristics of Launaea Sarmentosa.
Extraction
Ethanolic extract
The leaf of Launaea Sarmentosa were dried under shade at room temperature for seven days and powdered it by the use of grinder and were sieved through sieve no.40 to get the coarse powder (100gm) and was extracted with ethanol as solvent by Soxhlet apparatus and filtered then obtained extract was concentrated and stored in vacuum desiccator. The obtained yield was calculated. Then the ethanolic extract of Launaea Sarmentosa was subjected to qualitative and phytochemical analysis.8
Hydroalcoholic extract
The powder materials (200 g each) from the plants were separately soaked in of ethanol at room temperature for 7 days. The extracts were filtered through fresh cotton bed and finally with Whatman filter paper number 1. The filtrates were concentrated with a rotary evaporator at reduced temperature and pressure. These extracts were then stored in a refrigerator for further use.9
Determination of Physical Content10
Loss on drying
Weight of powder about 1.5 gm into a weighted flat and thin porcelain dish .Dry in oven at 1050 C till the weighing is constant. Cool in desiccator and take its final weight. The loss in weight on drying is noted as moisture.
Ash value
Total ash
Determination of ash value is used to determine quality and purity of a crude drug and it's the identity of it.
Procedure: Place about 2 gm of ground air dried powdered drug accurately weighed in a crucible heat on of 2 cm high flame heat till vapours almost cease. Allow the ash to cool in suitable desiccators for 30 min, and then weigh without delay. Calculate the percentage content of total ash in mg/g of the dried material.
A sample of 2gm was weighted and air dried in an exceedingly tarred silica dish. It was incinerated at a temperature not exceeding 450 0 C it was it absolutely was free from carbon, cooled and the ash was weighted and proportion of ash was calculated.
Acid insoluble ash
Method: The ash was obtained as per the method described above for total ash. The ash obtained was boiled with 25 ml 2M hydrochloric acid for 5 minutes. Filtered and also the insoluble matter was collected in Gooch crucible, washed with hot water, ignited, cooled, in a desiccator and weighed. The share of acid insoluble ash was calculated with air-dried the air-dried drug.
Water soluble ash
Method: The ash was obtained as per the method described above for total ash. The ash obtained was boiled for 5 minutes with 25 ml of water. Filtered and the insoluble matter was collected in a Grouch crucible, washed with hot water and ignited for 15 minutes at a temperature not exceeding 450° C. the weight of the collected insoluble matter was subtracted from the weight of the ash. The difference in that weight represented the water- soluble ash. percentage of water – soluble ash was calculated with the dry drug.
Extractive value determination
This method determines the number|the quantity of active constituents in given amount of medicinal plant material when it extract with solvents. The extraction of any crude drug with a specific solvent yields a solution which contains different phytoconstituents. The composition of these Phyto-constituents in that particular solvent yields a solution containg different phytoconstituents. The composition of these Phyto-constituents in that particular solvent depends upon the nature of the nature of the drug and solvent used.
Alcohol soluble extractive value
Accurately weight 5gm of air-dried crude drug was taken in closed flask and macerated with 100ml of 95th ethanol for 24hrs. Shaken frequently during the first 6hrs and filter rapidly taking precaution against loss of alcohol. 25ml of the filtrate was taken and evaporate to dryness on a water bath and complete drying at 105 0C. Then cool in desiccator and weighed. The percentage of alcohol soluble extractive value was calculated with reference to air dried drug.
Water soluble extractive value 11
5 gm of the air-dried drug, coarsely powdered have to be macerated with 100mi lf water closed flask for 24 hours, shaking frequently during the first 6 hours and allowing standing for 18 hours. Thereafter, filter rapidly taking precautions against loss of water, evaporate 25 ml of the filtrate to dryness in a tarred flat-bottomed shallow dish, dry at 1050 C and weigh. The percentage of water-soluble extractive value with reference to the air dried drug cease is calculated.
Phytochemical Study12
Test for Carbohydrates
Fehling’s test: 1 ml of Fehling’s A and 1 ml of Fehling’s B solutions mix then boiled for 1 min ,Added equal volume of test solution.Then heated in boiling water bath for 5-10 min. Brick red ppt was observed.
Benedict’s test: Same volume of Benedict’s reagent and test solution was mixed in test tube. Heated in boiling water bath for 5 min. Solution appeared in green colour.
Test for steroids
Salkowski reaction: In 2 ml of extract, added 2 ml of chloroform and 2 ml of conc. H2SO4. Shake well chloroform layer appears red and acid layer shows greenish yellow fluorescence’.
Test for flavonoids
A Sulphuric acid test: 2ml of extracts was treated with few drops of sulphuric acid solution. Formation of deep yellow colour indicates the presence of flavonoids.
Test for tannin and phenolic compounds
Lead acetate solution: Add 2 ml of extract in lead acetate solution then white ppt was observed for presence of tannins and phenols.
Dilute HNO3 Solution: Mix 2-3 ml of extract in dilute HNO3 then reddish to yellow colour taken as evidence for the presence of tannins and phenolic compounds.
Quantitative Analysis of Phytoconstituents
Total phenolic content13
Phenolic content was measured fusing the Folin-Ciocalteu colourimetric with some modification.1 ml of kenaf leaf plant extracts was taken into a test tube and poured with 2.5 ml of FolinCiocalteu phenol reagent (10%) and 2 ml of Na2CO3 solution (2%). The test tube was shaken and the solution was remained in the dark for two hours at room temperature. The blue-green color appearance formed in the incubated test tube indicated the presence of phenolics which was recorded at 765 nm. Thus, standard (0.1 mg/mL) was used as standard and serial dilutions were made with the range from 10 to 50 µg/mL. Result was presented as gallic acid equivalents (GAE) (mg/g of the extracted compound).
Total tannin content14
Formulation of ointment15
Evaluation of Ointment Formulation:16
Color and Odour: Color and Odor of all ointments was examined by visual examination.
PH: The PH of ointment formulation was determined by using digital PH meter.
Spread ability Test: Spread ability is expressed in terms of time in seconds taken by two slides.
Extrudability: Weight in grams required to extrude 0.5 cm ribbon of ointment in 10 sec was determined.
Viscosity: Viscosity of ointment was measured by using Brookfield viscometer with spindle 7.
Pharmacological Activity
Experimental animals
The protocol of the experiment (1942/PO/Re/S/17/CPCSEA/2020/01/07) was approved by Institutional Animal Ethics Committee (IAEC) of Pravara Rural College of Pharmacy, Loni and was conducted in accordance with permission from Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA).
Excision wound model
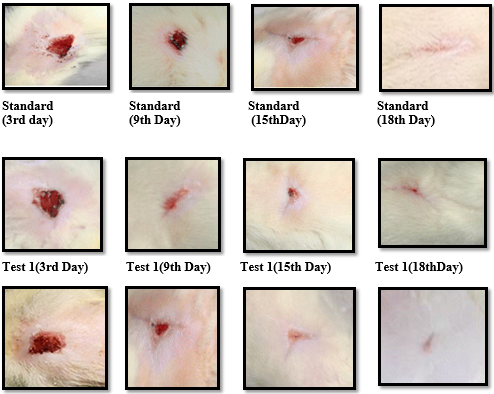
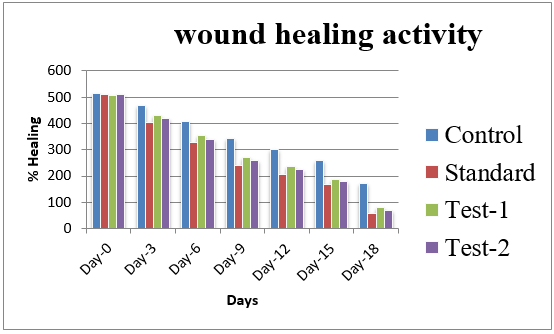
Excision wounds were used for the study of rate of contraction of wound and epithelization. Animal were anesthetized with ketamine and the hairs on the skin of the back, shaved with sterilized razor blade. A circular wound of about 300mm2 area and 2mm depth was excised rats, 5cm away from the ear. The entire wound was left open. This was done topically in all the cases. The wounds were traced on transparent tracing paper by permanent marker on the day of wounding and subsequently on alternate days until healing were complete. Wound area was measured on days 0, 3, 9, 12, 15, 18 for all the groups.17
Result and Discussion
Authentication
The plant was authentication by Department of Botany and Research Centre, Padmashri Vikhe Patil College of Arts, Science and Commerce, Pravaranagar on 4/12/2020 herbarium has been deposited in the department.
Pharmacognostic study
The Pharmacognostic study of leaves of Launaea Sarmentosa macroscopic, microscopic and physiochemical parameter were studied.
Macroscopic study
The macroscopic study is morphological description of the plant parts which are seen by naked eyes or magnifying lens.
Table 4
Morphological parameter
|
Sr. No |
Parameter |
Morphological character |
|
1. |
Color |
Green |
|
2. |
Odor |
Characteristics |
|
3. |
Taste |
Slightly bitter |
|
4. |
Size |
12-15cm long |
|
5. |
Shape |
Whip shape |
Table 5
Micro chemical test
Powder microscopic feature
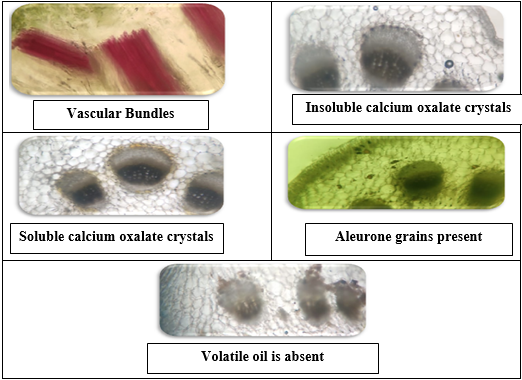
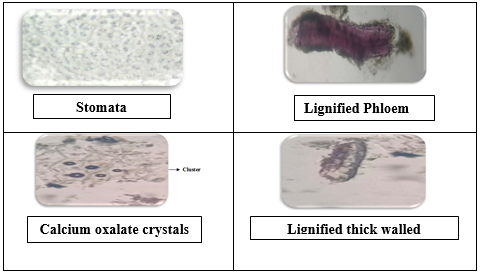
In the study of the powder microscopy of leaves of Launaea Sarmentosa the Upper epidermis was covered by cuticle and contains Lignified phloem. Lower epidermis was also covered by smooth cuticle and it also contains lignified thick walled fiber. Stomata were seen which is anisocytic type .vascular bundle is present and various identifying characters were observed under Motic electronic microscope and cluster types of Calcium oxalate crystals are present.
Extraction
The Ethanolic extract of Launaea Sarmentosa leaves was found to be 6.340 %. The Hydroalcoholic extract of Launaea Sarmentosa leaves was found to be 7.223%.Color, consistency, percentage yield of different extracts of leaves of Launaea Sarmentosa from successive solvent extraction.
Table 7
Totaltwig of extract
|
Extract |
Color of extract |
Extract of twig |
|
Ethanol |
Dark Green |
6.340% |
|
Hydroalcoholic |
Dark Green |
7.223% |
Table 8
Physicochemical parameter in Launaea Sarmentosa leaves
|
Sr. No. |
Parameter |
Value (%)w/w |
|
1. |
Total ash |
10 |
|
2. |
Acid insoluble ash |
2.5 |
|
3. |
Water soluble ash |
2 |
|
4. |
Loss on drying |
3.33 |
|
5. |
Alcohol Soluble extractive value |
8 |
|
6. |
Water Soluble extractive value |
16 |
Table 9
Preliminary phytochemical screening of extracts
Quantitative analysis of phytoconstituents
Total phenolic contents in Launaea Sarmentosa leaves extract was found to be 3.7mgGAE/gm
Total tannin contents in Launaea Sarmentosa leaves extract was found to be 20%
Table 10
Quantitative analysis of phytoconstituents
|
Sr. No. |
Phytoconstituents |
Value |
|
1. |
Total Phenolic Content |
3.7mgGAE/mg |
|
2. |
Total Tannin Content |
20% |
Formulation of Ointment:
Evaluation of ointment formulation
Color and Odor: Color and Odor of all ointments was examined by visual examination.
PH: The pH of ointment formulation was determined by using digital pH meter. 1gm of ointment was dissolved in 100 ml of distilled water and stored for two hours. The measurement of pH of each formulation was done in triplicate and average values were depicted.
Spreadability Test: Spread ability is expressed in terms of time in seconds taken by two slides. To flip out or from cream when placed in between the slides under the direction of certain load. Laser the time taken for separation of two slides, better the spread ability. It is calculated by using the formula. S = M x L/T, where, M = Weight tied to upper slide, S = Spread ability of formulation, L = Length of Glass Slides, T = Time taken to separate the slides.
Extrudability
A closed collapsible tubes containing ointments was pressed firmly at the cramped end. When the cap was removed, ointment extruded until pressure dissipated. Weight in grams required to extrude 0.5 cm ribbon of ointment in 10 sec was determined.
Viscosity
Viscosity of ointment was measured by using brookfield viscometer with spindle 7.
Table 11
Physicochemical evaluation parameters for ointments
Statistical Analysis
Data obtained were analyzed using one way analysis (ANOVA) and expressed as mean ± SEM. Differences between means were regarded significant at P <.0.01
Table 12
Effect of herbal formulation on healing of excision wound model
Conclusion
The research of selected plant for the formulation was literally proved for the therapeutic use of wound healing purpose.
In our daily routine we have major cuts, burns etc. Plant used in work was Launaea Sarmentosa were extracted by using ethanolic and hydroalcoholic solvent (7:3) was used for ointment formulation.
Physical parameters result revealed that all the values within acceptable limits. The herbal formulation shows significant wound healing activity.