Introduction
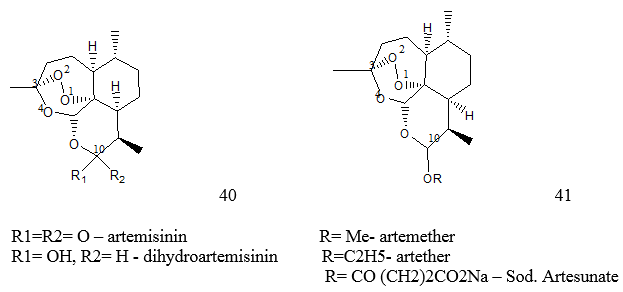
Artemisinin is a Chinese herb (Qinghaosu) that has been used in the treatment of fevers for over 1,000 years,1 thus predating the use of Quinine in the western world. It is derived from the plant Artemisia annua, with the first documentation as a successful therapeutic agent in the treatment of malaria is in 340 AD by Ge Hong in his book Zhou Hou Bei Ji Fang (A Handbook of Prescriptions for Emergencies).2 The active compound was isolated first in 1971 and named Artemsinin 40, It is a sesquiterpene lactone with a chemically rare peroxide bridge linkage. It is this that is thought to be responsible for the majority of its anti-malarial action. At present it is strictly controlled under WHO guidelines as it has proven to be effective against all forms of multi-drug resistant P. falciparum, thus every care is taken to ensure compliance and adherence together with other behaviours associated with the development of resistance. It is also only given in combination with other anti-malarials.3
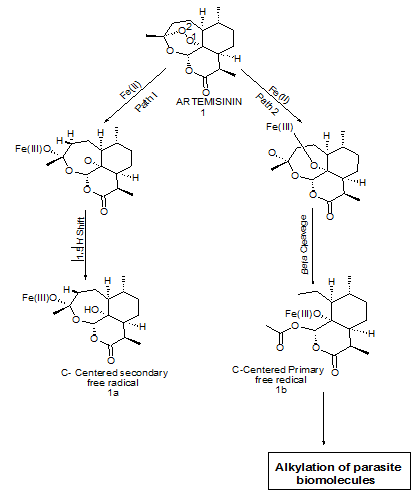
Artemisinin 40, has a very rapid action and the vast majority of acute patients treated show significant improvement within 1-3 days of receiving treatment. It has demonstrated the fastest clearance of all anti-malarials currently used and acts primarily on the trophozite phase, thus preventing progression of the disease. It is converted to active metabolite dihydroartemisinin that then inhibits the sarcoplasmic/endoplasmic reticulum Calcium ATPase encoded by P. falciparum. On the first day of treatment 20 mg/kg should be given, this dose is then reduced to 10mg/kg per day for the 6 following days. Few side effects are associated with artemisinin use. However, headaches, nausea, vomiting, abnormal bleeding, dark urine, itching and some drug fever have been reported by a small number of patients. Some cardiac changes were reported during a clinical trial, notably non specific ST changes and a first degree atrioventricular block (these disappeared when the patients recovered from the malarial fever).4
Artemether 41, is a methyl ether derivative of Dihydroartemisinin. It is similar to Artemisinin in mode of action but demonstrates a reduced ability as a hypnozoiticidal compound, instead acting more significantly to decrease gametocyte carriage. Similar restrictions are in place, as with Artemisinin, to prevent the development of resistance, therefore it is only used in combination therapy for severe acute cases of drug-resistant P. falciparum. It should be administered in a 7-day course with 4mg/kg given per day for 3 days, followed by 1.6 mg/kg for 3 days. Side effects of the drug are few but include potential neurotoxicity developing if high doses are given.5
Artesunate 41, is a hemisuccinate derivative of the active metabolite Dihydroartemisin. Currently it is the most frequently used of all the Artemisinin-type drugs. Its only effect is mediated through a reduction in the gametocyte transmission. It is used in combination therapy and is effective in cases of uncomplicated P. falciparum. In large studies carried out on over 10,000 patients in Thailand no adverse effects have been shown. Dihydroartemisinin 40, is the active metabolite to which Artemisinin is reduced. It is the most effective Artemisinin compound and the least stable.6 It has a strong blood schizonticidal action and reduces gametocyte transmission. It is used for therapeutic treatment of cases of resistant and uncomplicated P. falciparum. 4mg/kg doses are recommended on the first day of therapy followed by 2mg/kg for 6 days. As with Artesunate, no side effects to treatment have thus far been recorded. Arteether 41, is an ethyl ether derivative of Dihydroartemisinin. It is used in combination therapy for cases of uncomplicated resistant.
Arteflene- A synthetic derivative of Qinghaosu was evaluated extensively against various drug-sensitive and drug-resistant Plasmodium falciparum in vitro and P. berghei in mice. Experimentally arteflene proved to be a highly effective antimalarial drug.The suppressive and prophylactic properties were comparable to chloroquine and superior to Qinghaosu artemether and artesunic acid.7 It was consistently rather more active against drug-resistant than against drug sensitive strains of P. falciparum. Drug interactions in vitro and in vivo with Chloroquine, Mefloquine and Quinine revealed an additive to synergistic effect with arteflene.
Design of the Chemical Synthesis
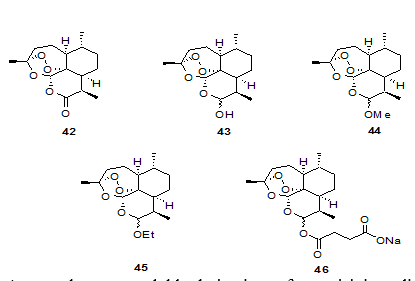
Discovery of artemisinin 42 in 1971 from Chinese traditional medicinal herb, Artemisia annua, has been a major breakthrough in malaria chemotherapy. It was isolated from the leafy portion of the plant. Artemisinin is a sesquiterpene lactone 1,2,4-trioxane with a unique structure and is active against both drug-sensitive and drug-resistant malaria.7 It is a fast acting blood schizontocide. To circumvent certain limitations associated with artemisinin, such as poor oil and water solubility, and high rate of recrudescence,8 several second generation semi-synthetic derivatives of artemisinin have been reported. Ether derivatives of artemisinin9, 10, 11 have the advantage of better oil solubility and are prepared by treating dihydroartemisinin 43 with an appropriate alcohol in the presence of an acid catalyst.12 Artemether 44 and arteether 45 are the two most important derivatives of artemisinin. Both of them show better oil solubility and improved activity and are currently in clinical use.13
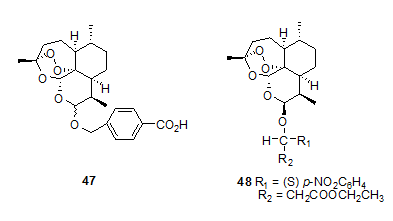
Among the water-soluble derivatives of artemisinin sodium salt of artesunic acid 46,14 exhibits potent antimalarial activity and can be administered intravenously. Artesunic acid, though currently used clinically for the treatment of complicated cases of malaria, is associated with major problem of poor stability in aqueous solutions due to rapid hydrolysis and extremely short plasma half-life.14 Artemisinin derivatives are fast acting blood schizontocides and are undoubtedly safe drugs for emergency treatment of severe multidrug resistant malaria15 but they should not be used for prophylaxis of malaria or treatment of mild attacks. Lin et al.16 have synthesized several water soluble and hydrolytically stable derivatives of 43. Among them artelinic acid 47 shows better in vivo activity. In their attempt to synthesize compounds superior to artelinic acid, they prepared a number of a-alkyl benzylic ether derivatives of dihydroartemisinin and tested them for their in vitro antimalarial activity.17, 18 Compound 48 was found to be the most active compound showing activity nearly 10-, 20-, 40- folds better than artemether, arteether and artelinic acid respectively.
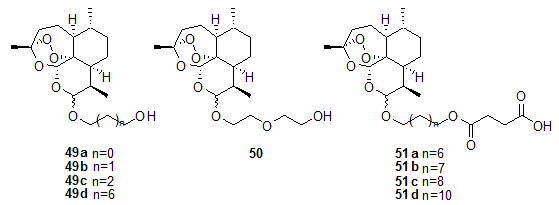
Singh et al.19, 20, 21 also reported the synthesis of hydrolytically stable derivatives of 43 wherein dihydroartemisinin and succinoyloxy moieties are joined through a linker of carbon chain.
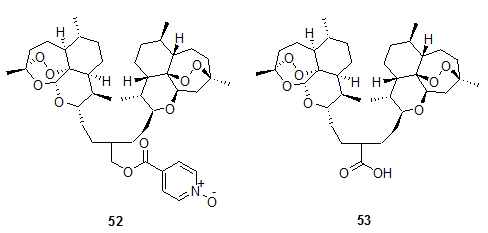
Among them compounds 49a-d and 50 were found to have activity comparable to -arteether. While hemisuccinates 51a-d showed activity comparable to artesunic acid. Posner et al.20, 21, 22, 23, 24 have prepared artemisinin derived dimers 52 and 53. In mice both 52 and 53 were more effective than clinically used sodium salt of artesunic acid.
However, artemisinin and its derivatives are associated with serious problems of high rate of recrudescence and limited availability from natural sources. With the establishment of the fact that artemisinin and its derivatives owe their antimalarial activity to the peroxide group present as 1,2,4-trioxane, there has been an increased interest in the synthesis and biological evolution of organic peroxides particularly 1,2,4-trioxanes. As a result several new methods for their preparation have been developed in the recent years. Following is a brief account of activity profile of 1,2,4-trioxanes prepared by major groups working in this area. Singh et al. 22 Have prepared several in vivo potent spiro 1,2,4-trioxanes of different prototypes and were the first to report in vivo potent synthetic 1,2,4-trioxanes. In the preliminary study on 6-arylvinyl trioxanes, compounds 54-57 showed activity at 30 mg/kg by intra peritoneal (i.p.) route against chloroquine sensitive P. berghei in mice but these compounds were poorly active against chloroquine resistant P. yoelii in mice when given by im route. Optimization of this lead furnished several compounds with high potency against multi-drug resistant P. yoelii in mice. Trioxanes 58a-d were as or more potent than arteether by oral route.
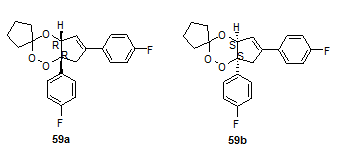
Jefford et al.23 Were the first to report in vitro antimalarial activity of simple 1,2,4-trioxanes. Among all the 1,2,4-trioxanes screened, cis-fused cyclopenteno trioxanes showed the most promising activity profile. Fenozan B07 (earlier Fenozan 50F), a difluoro 3,3’-spirocyclopenteno-1,2,4- trioxane 59 was the best compound of the series. To study the role of chirality on antimalarial activity, the same compound was resolved in to enantiomers 59a and 59b using chiral HPLC. But it was found that there was no difference in the activity profile of each enantiomer. Racemates and pure enantiomers have commensurate activities.
A large number of derivatives of artemisinin like 1,2,4-trioxanes, including ethers, carboxylate esters, phosphate esters, carbamates and sulfonates have been prepared by Posner and coworkers. Some of the compounds found active in vitro were also tested in vivo in mice model. Based on their antimalarial potency in mice, two trioxanes 60 and 61 were selected for biological evalution in Aotus monkeys infected with multidrug resistant (MDR) P. falciparum. Compounds 60 and 61 both were as effective as arteether against MDR P. falciparum in Aotus monkeys.24
On the basis of earlier observation by Jefford et al.25 that replacement of the C3-methyl by C3-phenyl improved antimalarial activity, various substituted C3-phenyl analogs of prototype 62, were also prepared by Posner et al.26 Some of these compounds 62a-e have shown promising in vivo activity. Trioxane alcohol 62a, acetate trioxane 62b and flouorobenzyl ether trioxane 62c are up to twice as potent as arteether whereas 62d and water soluble carboxylic acid derivative 62se were less active than arteether.27
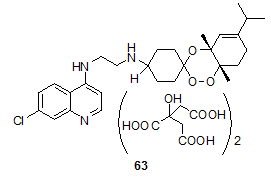
Zhou, W.S.28 have synthesized several trioxane-quinoline ‘hybrids’ (trioxaquines), some of which have shown promising activity profile in vitro and in vivo. Ascaridole-derived, trioxaquine 63, is the best compound of the series. It exhibits ED50 values of 5 mg/kg/day and 18 mg/kg /day by i.p. and p.o. routes respectively against P. vinckei in mice. This compound completely cleared parasitaemia in P. vinckei infected mice, without recrudescence, at an i.p. dose of 20 mg/kg /day.
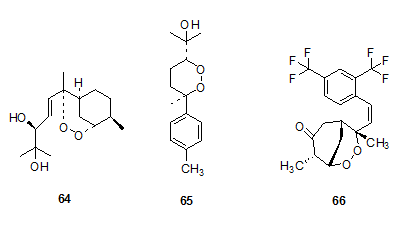
Yinghaosu A, Yinghaosu C and Arteflene given the success of the peroxide natural product artemisinin in drug development efforts, it was obvious for the researchers to explore the antimalarial properties of other naturally occurring peroxides. Two cyclic compounds containing a bisabolene skeleton have been isolated from the roots of the plant Yingzhao (Artabotrys uncinatus) which has been used as a folk treatment for malaria. Yingzhaosu A 64 was the first to be isolated, and the structure was proposed in 1979. Both compounds Yingzhaosu A 64 and Yingzhaosu C 65 have been synthesized and both are active antimalarials. Analogs have been prepared and some of them show significant antimalarial activity in vitro.[33-34] A particular example is arteflene 66, a synthetic antimalarial agent developed as an analog of Yinghaosu A.
The central core ring structure was shown to have inherent antimalarial activity. It is as active as artemisinin and has better chemical stability. In a mouse model, arteflene 66 was fast acting and a single dose lasts significantly longer and results in much lower recrudescence than with artemisinin. The pharmacokinetics and metabolism of Arteflene ,66 have been studied in animal models and in humans. Like artemisinin, the compound has low human toxicity and seems to interect with specific parasite proteins. In preliminary clinical trials, Arteflene was found effective in reducing clinical symptoms and was well tolerated.
Conclusion
In this paper, author explains the possible routes for the chemical synthesis of trioxane derivatives in reference Artemisinin. With the literature on Artemisinin compounds, the possibility of synthesis of trioxane made easy due to heterocyclic nucleus in Artemisinin. Artemisinin have two to three oxygen in its structure whereas trioxane having three oxygen in its all derivatives. On the basis of those facts, it is clearly see that trioxan derivatives wil l also show potent anti malarial activity. A variety of drugs in market today posses trioxane ring and ongoing research is focused on developing newer anti malarial in which thrioxne can play major role. Hence, it can be concluded that this paper suggests all the strategies for the design of synthesis of trioxane also development of potent anti malarial agents over Artemisinin through various modifications.