Introduction
Calcium is the most abundant mineral and the fifth-most abundant element in the human body. Calcium is an essential mineral to support bone health and serves as a major therapeutic intervention to prevent calcium deficiency disorder.1 It is an important micronutrient widely believed to affect bone health and human metabolism. Calcium enters the circulation through food or calcium supplements, and a dynamic balance is maintained between blood and bone calcium. Calcium deficiency can cause conditions like osteoporosis, rickets, epilepsy, and anemia.2, 3
Calcium ions play a vital role in the physiological and biochemical processes of organisms and cells: in signal transduction pathways where they act as second messengers; in release of neurotransmitter from neurons; in contraction of all muscle cells; as cofactors in many enzymes as well as in fertilization. The Ca2+ ion acts as an electrolyte and is vital to the health of the muscular, circulatory, and digestive systems; is indispensable to the building of bone; and supports synthesis and function of blood cells. For example, it regulates muscle contraction, nerve conduction, and blood clotting. As a result, intracellular and extracellular calcium levels are tightly regulated by the body. Calcium can play this role because the Ca2+ ion forms stable coordination complexes with many organic compounds, especially proteins. More than 99% of calcium in the body resides as hydroxyapatite, a calcium phosphate molecule in the skeleton.4
A well-balanced, carefully planned, plant based diet can provide adequate calcium to the body. Not consuming plant based foods containing high amounts of calcium may increase the risk for impaired bone mineralization and bone fractures in people. Adequate calcium intake is the key to bone health, which appears to be irrespective of dietary preferences.5 Some plant-based calcium sources include tofu, mustard and turnip greens, bok choy, and kale. Some other plants contain calcium that is bound to oxalate and therefore is poorly absorbed.6 Foods rich in calcium include dairy products, sardines, salmon, soy products, kale, and fortified breakfast cereals.7, 8, 9
Types of calcium supplement
There are different types of supplements. Elemental calcium is a pure mineral, but in the natural form, it exists with other compounds. Supplements contain varied proportions of calcium compounds. For example:
Calcium carbonate contains 40% elemental calcium,
Calcium lactate contains 13% elemental calcium,
Calcium gluconate contains 9% elemental calcium and
Calcium citrate contains 21% elemental calcium10
Calcium from plant sources may be better than calcium from the milk or other animal products, since animal proteins tend to leach calcium from the bones. Further, vegetables may also provide vitamins and minerals that exert beneficial effects on bone.11
Soybeans are rich in both oxalate and phytate, yet soya products have relatively high calcium bioavailability. In contrast, dried beans which are also rich in phytate, have lower calcium bioavailability.12 The natural sources of readily bioavailable calcium are rare. However, the mineralized remains of certain red algae are rich sources of bioavailable calcium.13 Red algae belong to a group of multicellular aquatic plants that are photoautotrophic in nature.14 One such example would be Lithothamnion calcareum, the skeletal remains of which contain calcium carbonate (~32% calcium by weight) and 72 other trace elements.13, 15 This marine algae accumulates calcium carbonate in its cell walls in the form of crystals.
Red coral algae precipitate calcium by ‘trans-calcification’ mechanism. This mechanism is enzymatically driven, in which HCO3- present in seawater are converted to carbon dioxide CO2 by an external enzyme- carbonic anhydrase. This in turn produces CO3- which is used in calcification in the form of CaCO3.16 This calcium carbonate derived from marine red algae is used as a plant based calcium source in many countries due to its bioavailability and beneficial anabolic effects on bone calcification.17
Calcium is a common constituent of multivitamin dietary supplements, but the composition of its complexes in supplements may affect its bioavailability. This varies by solubility of the salt involved as calcium citrate, malate, and lactate are highly bioavailable, whereas the calcium oxalate is less. Other calcium preparations include calcium carbonate, calcium citrate malate as well as calcium gluconate. The intestine absorbs about one-third of calcium eaten in the form of free ion, and plasma calcium level is then regulated by the kidneys.
In the present study, we have used different analytical techniques to identify the source of calcium, whether it was from a plant based source or whether it was from a synthetic source.
Materials and Methods
Plant based Calcium was from Lithothamnion calcareum which is red algae and manufactured by Marigot Ltd., Strand Farm, Currabinny, Co. Cork. Ireland. Synthetic Calcium carbonate used in the present study was manufactured by Saurashtra Solid Industries Pvt. Ltd., 124-25 Near Dabka Gas Station, Padra Jambusar Road, Gavasad, Tal-Padra, Dist- Vadodara, Gujarat- 391430.
Samples of Calcium were subjected to instrumental analysis like SEM, ICP-OES and XRD. Scanning Electron Microscope (SEM) is a type of Electron Microscope and the method known as SEM analysis is used very effectively in microanalysis of solid inorganic materials. It produces images by scanning the surface with a focused beam of electrons which interact with atoms in the sample and produce signals that give information about the surface topography and composition of the sample. Scanning of electron beam is done followed by combining its position with the intensity of the detected signal to generate an image. In the most common SEM mode, atoms excited by the electron beam emit electrons which are detected using a Everhart-Thornley detector. The number of secondary electrons that can be detected, and thus the signal intensity, depends on specimen topography. Some SEMs have the ability to achieve resolutions greater than 1 nanometer (nm).18
In the present case, both samples were analysed using SEM as per below mentioned two different settings:
EHT=20.00 kV, Signal A= SE1, WD=7.5 mm, Mag=8.28 KX, scale=2µm
EHT=15.00 kV, Signal A= SE1, WD=8.0 mm, Mag=2.03 KX, scale=10µm
Inductively Coupled Plasma-Optical Emission Spectroscopy (ICP-OES) is an analytical technique that identifies the composition of elements present in different samples. The key strengths of ICP-OES include the identification of the types and ratios of elements in complex samples. The type and relative amount of each element present within the complexity of a compound is detected by the generated signals. ICP-OES is used in the analysis of complex samples, and in applications such as analyzing trace elements in the human brain, determining the chemical composition of electronic cigarettes and assessing the purity of pharmaceutical compounds. 19
In the present case, 1 g of plant based calcium sample was digested with conc. HF followed by 10 ml conc. HNO3. After complete digestion, solution was filtered and volume made upto 25 ml with 5% HNO3. Then this sample was injected in ICP-OES and calcium was measured at 396.84 nm. Similar procedure was carried out for synthetic calcium.
X-Ray Diffraction Analysis (XRD) studies the crystalline structure, atomic arrangement, crystallite size and imperfections in the structure. Compared to amorphous material, a crystal has well-defined properties like melting point, solubility and IDR. These parameters should be known in order to control the final product. The analysis of XRD works on constructive interference of monochromatic X-rays. The X-rays produced by a cathode ray tube are filtered to produce monochromatic radiation. They are collimated to concentrate and directed toward the sample. Interaction of the incident rays with the sample produces constructive interference when the conditions satisfy Bragg’s Law (nλ=2d sin θ). The relation between the diffraction angle, wavelength of electromagnetic radiation, and the lattice spacing in a crystalline sample is given by this law.20
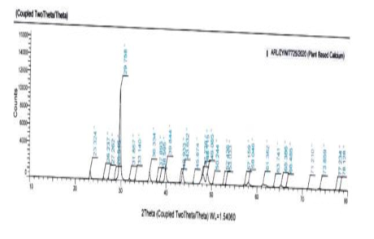
In the present case, 1 gm of plant based calcium sample was subjected to XRD analysis (Range 0 to 80) at wL=1.54060 and 2θ angle versus Intensity was determined.
Results and Discussion
Inductive coupling plasma- optical emission spectroscopy (ICP-OES)(Table 2 )
Powder X-ray diffraction (PXRD)Table 3, Table 4 Figure 5, Figure 6
Table 3
Synthetic Calcium XRD
Table 4
Plant Calcium XRD
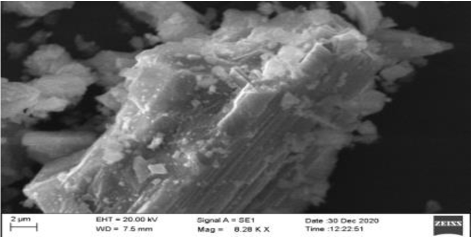
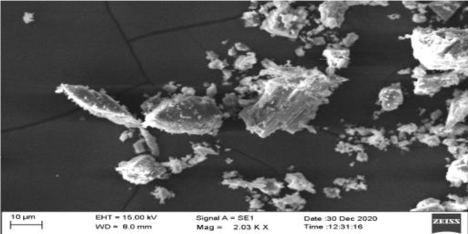
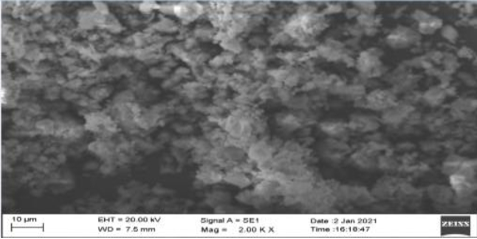
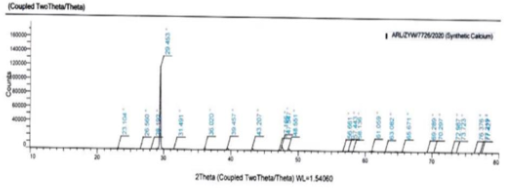
The ICP-OES study results showed the presence of Boron and Magnesium along with Calcium in both plant-based as well as synthetic sources of calcium. Inspection of results from SEM revealed that plant based calcium were smaller, even sized crystals (isotropic) as compared to synthetic calcium crystals which were uneven (anisotropic). Further, XRD results revealed plant based calcium to possess two 2θ values, whereas in case of synthetic calcium, it showed a single 2θ value.
Elble et al., 2011, highlighted the importance of smaller sized crystals of calcium carbonate stating that they are better absorbed in the body compared to larger sized crystals.21 The different mineral phases of calcium carbonate - calcite, aragonite, vaterite exhibit a high solubility which makes these minerals bioavailable and bioresorbable.22 This may be one of the defining factors of plant based calcium being more bioavailable and better absorbed as compared to synthetic form which exists in a single crystal structure.
A study carried out in female mice involved the analysis of the effects of a plant based mineral rich extract from Lithothamnion calcareum on their bone structure and function when supplemented with a high fat Western style diet. Ideally such a high fat diet interferes with the bioavailability of minerals. The results, however, revealed that the mice fed with such a diet and supplemented with the mineral rich extract had better bone structure and function compared to the ones on a low fat chow diet.23 Another study involved the use of the calcium rich, multi-mineral complex derived from Lithothamnion in ovariectomised rats suffering from osteoporotic bone loss. The effect of this complex was studied against the effect of calcium carbonate. The multi-mineral complex was shown to have a positive effect on the bone structure, composition and mechanical properties as compared to calcium carbonate.24
The above mentioned effects of such multi-mineral complex from marine algae- Lithothamnion can be attributed to the presence of calcium, manganese, copper, zinc and many other minerals present in the supplement that improve bone health and manage osteoporosis by increasing the mineralisation of osteoblasts as revealed in the study by Gormon et al., 2011.24 Studies have also shown that the osteogenic potential of this multi-mineral algal complex is enhanced by additional supplementation of Vitamin D3.25
The bioavailability of active absorbable algal calcium against that of calcium carbonate was evaluated with the use of stable isotopes and measured by dual stable isotope method. The study carried out in 10 postmenopausal women revealed the fractional absorption of algal calcium was 1.57 times higher than that of calcium carbonate.26
OZiva is a leading clean plant based nutrition brand which develops products that ensure better bioavailability and absorption of plant based nutrient components for health benefits.27
Conclusion
SEM: On the basis of SEM, Figure 1, Figure 3 are of Synthetic Calcium and Plant Calcium respectively. The Synthetic calcium Crystal is bigger in size than the Calcium crystal from plant source. It indicates that the Calcium Crystallization in plants is a slow process due to which there are a number of small sized crystal lattice structures. Further the shape of the plant based Calcium crystal was Isotropic whereas that of the synthetic Calcium crystal was anisotropic.
ICP-OES: Based on the Elemental analysis, it is clear that both the Plant as well as Synthetic Calcium consist of the same elements that is- Boron, Magnesium and Calcium. The amount of Boron was approximately 7 times higher in case of plant based calcium as compared to synthetic calcium. Further, the amount of magnesium was approximately 2 times higher than calcium.
XRD: The XRD results clearly showed that both the Plant and Synthetic calcium are Crystalline in nature. Plant based Calcium is having one 2θ at 29.75 (which is similar to Vaterite) and another 2θ at 45.9 (which is similar to Aragonite). In case of synthetic calcium, 2θ was obtained at 29.45 (which is similar to Vaterite). 28, 29
Both natural and synthetic Calcium were distinguished using SEM and XRD techniques
Anisotropic materials are more common in nature than isotropic materials due to the large variation in atomic orientations. Anisotropic crystals have many refractive indices whereas isotropic crystals have only one.
Thus, better absorption in plant based calcium may be due to smaller size of crystals in two crystalline forms (vaterite and aragonite), as compared to synthetic calcium crystals which are larger in size and in the form of vaterite crystals.
However, further in vitro and in vivo studies will be required to prove better Pharmacokinetics and Bioavailability proof of concept of plant based calcium source as compared to synthetic calcium source.