Introduction
Nanotechnology is an extremely convincing technology with its approaching medical properties in early disease detection and treatment. At present, there is a growing concern in the research of nano-metals as these signify an intermediate dimension between atoms/molecules and bulk particles.1 Among metal nanoparticles, iron oxide has received more attention because of its variety of scientific and technological applications such as antimicrobial activity2, biosensor3, food preservation4, ferrofluids, magnetic resonance imaging, magnetic refrigeration, magnetic storage media, cell sorting, targeted drug delivery, and hyperthermia cancer treatments.5, 6, 7
The size of nanoparticles makes them an attractive applicant for in vivo and in vitro biomedical research. The incorporation of nanoparticles in the medical field has led to their special use in the field of sensing, drug delivery, imaging, and artificial implants. An interesting new approach for their investigation in the medical field is their application in antimicrobial activities that aim at drug-resistant and highly pathogenic microbes.8
There is also an increasing interest in the application of Iron and other metal nanoparticles in blood platelet sanitization. Ag+ ions may slow down some imperative transport processes such as succinct and phosphate uptake, by contacting the cellular oxidation process in the respiratory string. The high absorption of Ag+ not only causes toxicity but may also lead to electrolyte imbalances in patients.9
In recent research, it has been shown that the better antibacterial properties of iron nanoparticles (Fe NPs) are dependent upon the high surface area and high volume of nanoparticles that allow proficient bacterial disinfection.10, 11 Iron nanoparticles (Fe NPs) have a larger surface area compared to silver so that they possess better antimicrobial properties as compared to Ag+ ions.12, 13, 14, 15 Despite this, in an aqueous solution zero-valent iron nanoparticles (Fe NPs) can inactivate E. coli. Which increases the interest of iron nanoparticles in antimicrobial activity. In the deficiency of oxygen, iron nanoparticles (Fe NPs) show high antimicrobial activity analogous to that of silver nanoparticles (Ag NPs). The strong antibacterial activity of iron nanoparticles (Fe NPs) implies that Fe NPs can serve as a cost-effective biocide for many of the applications in which silver is being used.16 In the case of antibacterial activity, iron nanoparticles (Fe NPs) involve the release of Fe ions on the biological obligatory site that hold upon to the surface of the targeted atom and developed a bacterial interaction with the NPs that starts from the surface layer.17, 18, 19
Materials and Methods
Green method
Fabrication of Fe NPs using S oleracea aqueous leaf extract
Fe NPs were fabricated by using fresh leaves of S. oleracea extract as a reducing agent. For the fabrication of Fe NPs, fresh leaves of S. oleracea were washed, dried at room temperature, and cut into small pieces. Dried-out leaves are crushed into powdered form and from which 4g was mixed in 50 ml of double distilled water and then boiled for 15 min at 100oC. Filter the extract and stored it at 40 C. Furthermore, 10ml of S. oleracea extract was added into 10ml aqueous solution of 1.0 mM ferric chloride. The mixture was boiled for about half an hour and after which black color was observed, representing the fabrication of Fe NPs.20
Chemical reduction method
Fabrication of Fe NPs using NaBH4 as reducing agent
In a three-neck, round-bottom flask 0.1M FeCl3.6H2O (4.1703 g) and 0.05M EDTA (3.7224 g) were mixed in 100 ml Milli-Q water by using appropriate mixing. Then the solution of NaBH4 (0.75M NaBH4 (2.837 g) in 100 ml Milli-Q water) was added dropwise into the above solution. Slowly the solution changed to black color.21, 22, 23
Results and Discussion
Characterization of Fe NPs
Analysis by ultraviolet-Visible spectra
An ultraviolet-Visible spectrum is an effectual technique that illustrates the development of varieties of metals in the creation of colloidal M NPs. As exposed in Fig. 1a the optical absorbance Plasmon band of the Fe NPs using S. oleracea extract in the colloidal suspension is about 267 & 229. In the case of optical absorbance Plasmon band of Fe NPs using chelating Agents (EDTA) Fig-1b in the colloidal suspension is about 273 & 224. In both cases, the black color appeared due to the formation of Fe NPs.
Zeta size measurement
The zeta potential value (21.9) shows the stability of Fe NPs using S. oleracea extract and confirms the size of Fe NPs particles to be 80.37(d. nm). On the other hand, the zeta potential value (-21.2) of Fe NPs using classical reduction method with EDTA and found the size of Fe NPs to be 51.37 (d. nm) (Fig- 2: A&B).
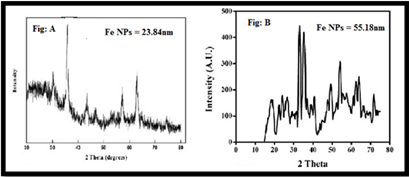
XRD of Fe NPs by chemical reduction and green method
The aqueous solution of Fe NPs was frequently centrifuged at 10,000 rpm for 30 min, redispersed with distilled water, and lyophilized to get pure Fe NPs pellets. The Debye-Scherer principle has been used to evaluate the standard particle size that could be understood as:
D = 0.9λ/ β Cosθ
An average dimension of Fe NPs was found in the green method is 55.18 nm and in the chemical reduction method 23.84 nm by XRD methods. (Fig-3: A&B)
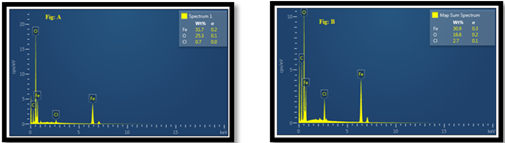
EDX of Fe NPs by chemical reduction and green method
EDX is Energy Dispersive X-Rays (aka EDAX - energy dispersive x-ray analysis) and is reliant on the atomic mass of the elements being detected. In the fabrication of Fe NPs in the chemical reduction method the wt% value of Fe is 31.7, O is 25.3 and Cl is 0.7 and in the case of the green method the wt% value of Fe is 30.9, O is 16.2 and Cl is 2.7. The peak of Fe presents in the spectrum shows the fabrication of Fe NPs.
FE SEM analysis of Fe NPs
The FE SEM image shows that the size of indusial Fe NPs was found to be around 50-60 nm using S. oleracea extract confirms the size of Fe NPs particles to be 51.22 (d. nm). On the other hand, the Fe NPs using the classical reduction method with EDTA was found to be 47.97(d. nm) (Fig-5)
In-vitro antibacterial studies of nanoparticles
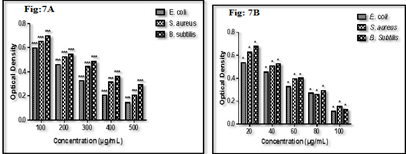
The iron nanoparticles produced by the liquid phase reduction technique were subjected to the in-vitro antibacterial activity of iron nanoparticles aligned with gram-negative (E. coli) and gram-positive (S. aureus and B. subtilis) respectively. Iron nanoparticles showed excellent and dose-dependent activity when compared to a standard antibiotic. The effects obtained after 24h incubation are presented in table 1 and fig6. Test compound the highest region of prohibition at 500μg/mL was 21 ± 0.64 mm as compared to standard ampicillin.
Table 1
The results of inhibitory antibacterial activity of iron nanoparticles against different bacteria
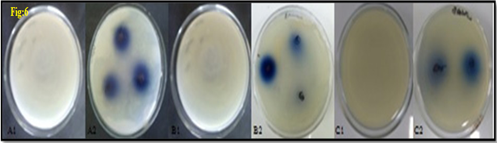
Figure 6
Zone of inhibition of test compound and standard against three different bacterial strains as compared to standard antibiotic at concentration (80μg/mL) (A1) E. coli control;(A2) Treated nanoparticles (300 & 350 μg/mL) on E. coli; (B1) S. aureus control; (B2) Treated nanoparticles (200 & 300 μg/mL) on S. aureus; (C1) B. subtilis control; (C2) Treated nanoparticles (400 μg/mL) on B. subtilis.
Tube dilution assay
In tube dilution assay, prepared nanoparticles showed significant antibacterial activities against targeted bacterial strains. IC50 of test compound was found to be 350 μg/mL, 300 μg/mL, and 400 μg/mL against bacterial strains compared with IC50of standard ampicillin was found to be 40 μg/mL,50 μg/mL, and 50 μg/mL against pathogenic E. coli, S. aureus and B. subtilis respectively (Fig. 2a &2b). Test compounds have shown better activities against B. subtilis and it was observed that it showed maximum ZOI and minimum IC50 (300 μg/mL) in both in vitro models. It indicates that it can be useful in the management of various B. subtilis mediated infections. The results of biological activity are revealed that iron nanoparticles found active against both strains but slightly better against gram-positive strains. The findings of the study were found in agreement with previously published reports (Behera et al. 2012).
Conclusion
Today, iron nanoparticles’ production with the help of plant materials or in an eco-friendly manner is considered attractive. The attrition of metallic ions into base metals by the biological method is a rapid and more efficient method than conventional methods. This method is eco-friendly and can be conducted willingly at room temperature and is also amplified easily. The reducing agents drawn in contain various water-soluble metabolites and coenzymes. The reward of holding green methods for nanoparticle amalgamation has increased the demand and the curiosity of analyzers to examine the procedures for uptake of metal ions and biological reducing methods by plants. Moreover, green synthesis generates nanoparticles that are highly stable and have narrow size distribution with high discrepancy.