Introduction
Parkinson’s disease (PD) is a second most common neurological disease after Alzheimer’s disease. The main objective of first line PD drugs were the movement dysfunction. In the L -dopa-treated PD patients psychotic systems are seen commonly as side effect. Early therapies for PD psychosis included lowering anti-PD dosages or prescribing a conventional antipsychotic, both of which have been shown to increase PD symptoms. Pimavanserin tartrate is an atypical anti-psychosis used in treatment of Parkinson’s disease.1
Pimavanserin tartrate is a salt that is the hemitartrate salt of pimavanserin. It is chemically named as (2R, 3R)-2, 3-dihydroxybutanedioic acid; 1-[(4-fluorophenyl) methyl]-1-(1-methylpiperidin-4-yl)-3[[4(2methylpropoxy) phenyl] methyl] urea.2
To treat L -dopa-induced psychosis in Parkinson’s disease and also for low-dose risperidone treatment in schizophrenia it act as inverse agonist at 5-HT 2A.3
The literature survey shows research on analytical study of PMT. The degradation study of PMT by identification and characterization of degradants,4 bioanalytical study on UPLC/Ms method in Rat,5 Quantification in bulk and formulation by high performance liquid chromatography.6
There wasn't even a publication on high performance thin layer chromatography (HPTLC) for determining PMT in bulk or in-house tablet formulation till now. So we perform this method to give precise, accurate and sensitive result by HPTLC method as per ICH guidelines.
Materials and Methods
Pure Pimavanserin tartrate provided by MSN Laboratories PVT. LTD., Hyderabad, India as a gift sample. Analytical reagent methanol, chloroform were of HPLC grade procured from Rankem, avantor performance materials India Ltd. Thane, India.
Equipment
The sample was transferred to the plate using a 100 µl sample syringe (CAMMG Hamilton, Switzerland) by a Linomate V sampler applicator with continuous nitrogen gas flow (CAMMG). The plate used for application was Aluminium plate coated with silica gel G60 F254 plates (10×10 cm, 6mm) (E. Merck, Darmstadt, Germany; supplied by Merck India, Mumbai, India) as stationary phase. The plate was prewashed with methanol in twin through chamber and dried at 115℃ for 5 min before chromatography. The applied plate was placed for development in automatic developing chamber (ADC 2 CAMMG) after mobile phase saturation.TLC scanner 3 was used to scan the developed plate, which was connected to the scanning software of the winCAT data processor software (version 1.4.10).
Stock sample preparation
To prepare 1000 µg/ml, a standard stock solution was made by accurately weighing 10 mg of PMT and transferring it into a 10 ml volumetric flask, which was then diluted with methanol up to the mark. A 0.2 µ membrane filter was used to filter solution.
Sample preparation for analysis
To prepare 10 µg/ml solution of sample by withdrawing 0.1 ml from standard stock solution in 10 ml volumetric flask and diluted with methanol.
In-house tablet formulation preparation
The in-house tablet was manufactured with a weight of 150 mg by combining 10 mg of medication with 8 mg of magnesium stearate, 8 mg of pregelatinized starch, and microcrystalline cellulose. All mixture was blended with morter and pestle. Compressed by using mini tablet compressor and make 20 tablet of 150 mg weight.
Validation of method
As per ICH guidelines the developed method was validated. The method validation parameter as per guidelines are linearity, precision, accuracy, ruggedness, detection of limit and quantification.
Linearity
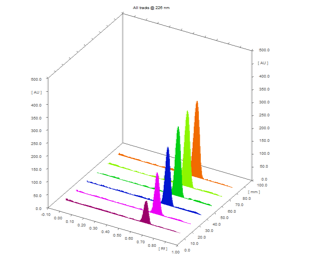
The linearity was evaluated by taking six sets of aliquots ranging 700 to 4200 ng concentration of pimavanserin tartrate from standard stock solution. These concentration applied on plate and tested through plotting peak area versus drug concentration. The linearity of curve was observed.
Precision
The intra-day, inter-day and repeatability parameter studied as in precision. The sample application for calculation in intra-day and inter-day as 1400 ng/band, 2100 ng/band, 2800 ng/band.
Accuracy
The term accuracy refers to the degree to which the measured and true values are similar. The accuracy was measured by spiking of pre-analyzed sample with 80%, 100%, 120% extra pimavanserin standard. To study drug recovery in formulation at different level and re-analyzed the sample.
Robustness
The robustness of the HPTLC method was tested by incorporating small changes in the composition of the mobile phase, chamber saturation time, and slight change in the solvent migration distance, the results were examined.
Limit of detection and limit of quantitation
In analytical method lowest amount of sample that can be detected known as Limit of Detection (LOD) whereas the lowest amount of sample that can be quantified known as Limit of Quantification (LOQ). The detection was calculatedby (3.3 x A.S.D)/b and Quantification limit was calculated by (10 x A.S.D)/b where, b, correspond to graph slope.
Result and Discussion
The literature survey of PMT shows that the few paper on HPLC and HPTLC methods. The objective of study to accurate, precise, cost-effective study by TLC technique for the determination of PMT.
Optimization or development of TLC
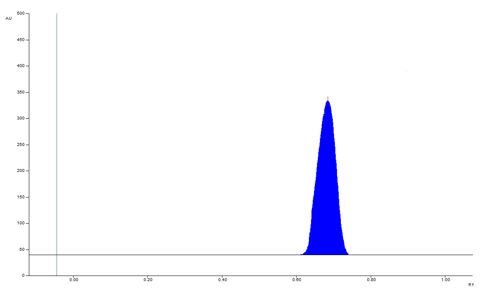
The selection of mobile phase was performed on polarity index of solvent. The slightly tailing was observed in methanol: chloroform (4:6 v/v). For good resolution and precise result tri-ethylamine introduced. The mobile phase of methanol, chloroform, trimethylamine (4:6:0.1 v/v/v) for PMT. It shows symmetric and sharp peak with RF value of 0.69 ± 0.03 (fig. 1). The mobile phase containing chamber was saturated for 20 min at room temperature and plate activated at 5 min.
Study of calibration curve
The developed method of linearity was linear in range of 700 – 4200 ng/band (fig. 2). It shows the good linear relationship with applied sample concentration. The co-relation coefficient was found to be 0.998 with linear equation of 0.5682x + 1870.6 (table 1).
Precision
The precision was described by relative standard deviation (RSD %). The intraday and inter-day precision RSD value was found to be 0.59-1.10 and 0.80-1.7 respectively (table 2).
Accuracy or recovery
The recovery studies by analytical technique of pre-analyzed sample spiked with standard PMT is known as recovery or accuracy. The results of accuracy were shown in table. The % recovery was found to be99.98 – 100.19 (table 3).
Robustness
For developed HPTLC method, the standard deviation and percentage of RSD of the robustness parameter was calculated. The results of robustness given in (table 4)
Table 4
Result of robustness
Conclusion
The developed TLC/Densitometry method is a simple, accurate, and repeatable quantitative analysis for estimating Pimavanserin Tartrate in-house tablet formulation. The procedure has been validated as per ICH guidelines Q2 (R1). The method is precise and there is no intervention from any of the sample in the study. It can be concluded that the system established offers many advantages such as quick, cost-effective, simple mobile phase and simple preparation measures, more sensitive and comparative short run time.
Source of Funding
The authors would like to thank the ‘Department of Science and Technology-FIST, Govt. of India’ (Grant No. SR/FST/College-2018/268) for funding the project.
Acknowledgement
The authors would like to thank the ‘Department of Science and Technology-FIST, Govt. of India’ (Grant No. SR/FST/College-2018/268) for funding the project. The authors are thankful to Dr. S. J. Surana Principal R. C. Patel Institute of Pharmaceutical Education and Research, Shirpur, for providing essential laboratory facilities and grateful to MSN Laboratories PVT. LTD., Hyderabad, India for providing Pimavanserin tartrate as a gift sample.