Introduction 1, 2, 3, 4, 5
Gastroretentive drug delivery system
Gastro-retentive drug delivery is an approach to prolong gastric residence time, thereby targeting site-specific drug release in the upper gastrointestinal tract (GIT) for local or systemic effects. Oral route of drug delivery has known from decades as the most widely used and preferred amongst all the routes that have been explored for the systemic drug delivery. The tremendous popularity of the oral route is mainly based on the convenience it offers to patients. However oral route has distinct failure in the delivery of oral sustained release dosage forms (OSRDF).
All OSRDF have limited applications because these systems cannot remain in the vicinity of absorption site in gastrointestinal tract (GIT) till complete release of active moiety. To improve the performance of OSRDF, scientists have discovered a new concept in drug delivery i.e. GRDDS. An optimum GRDDS can be defined as a system which retains in the stomach for a sufficient time interval against all the physiological barriers, releasing active moiety in a controlled manner, and finally metabolized in the body. Over the last two decades, numerous GRDDS have been designed to prolong GRT. The major objective is to minimize the drawbacks associated with existing OSRDF and optimizing therapy coupled with substantial patient comfort.
A GRDDS can be also being a useful tool in delivery of drugs that are primarily absorbed in the duodenum and upper jejunum or those that have an absorption window in the GIT.
These systems are also appropriate for drugs which are locally active in the gastric mucosa in the stomach, for example antibiotic administration for helicobacter pylori eradication and in the treatment of peptic ulcer and gastritis. Drugs that are less soluble in or are degraded by the alkaline pH may get benefit by being incorporated in GRDDS for prolonged gastric retention thereby improving the oral bioavailability and therapeutic efficacy by possible reduction of dose size.
Floating drug delivery system 6, 7
A floating drug delivery systems floats in the gastric juice without affecting the gastric emptying rate.
This helps to increase the drugs gastric residence time and reduces the variability in bioavailability.
Floating systems or hydro dynamically controlled systems are low-density systems that have sufficient buoyancy to float over the gastric contents and remain buoyant in the stomach without affecting on the gastric contents; the drug is released slowly at the desired rate from the system. After release of drug, the residual system is emptied from the stomach.
Mechanism of floating system 8, 9
An increased gastric retention time and better control on the fluctuation in the plasma drug concentration. However, besides a minimal gastric content needed to allow the proper achievement of the buoyancy retention principle a minimal level of floating force is also required to keep the dosage form reliably buoyant on the surface of the meal.
Literature Review 10, 11, 12, 13, 14, 15, 16, 17, 18, 19
Tripathi julu, Thapa Prakash (2019) in recent years, many attempts have been made to enhance the drug bioavailability and therapeutic effectiveness of oral dosage forms, various gastro retentive drug delivery systems (GRDDS) have been used to improve the therapeutic effcacy of drugs that have a narrow absorption window, they are unstable at alkaline pH soluble in acidic conditions, and active locally in the stomach. In this review, we discuss the physiological state of the stomach and various factors that affect GRDDS. Recently applied gastrointestinal technologies such as expandable, super porous hydrogel; bio/Mucoadhesive, magnetic, ion-exchange resin; and low and high-density-systems have also been examined along with their merits and demerits. The significance of in vitro and in vivo evaluation parameters of various GRDDS is summarized along with their applications. Moreover, future perspective son this technology are discussed to minimize the gastric empty in gratein both the fasted and fed states. Overall, this review may inform and guide formulation scientists in designing the GRDDS.
Sarkar Rao K et al., (2015) Losartan potassium is angiotensin type-II receptor antagonist, for the treatment anti-hypertension. The purpose of this investigation is to improve bioavailability, gastro residence time and to reduce frequency of administration by preparing a gastro-retentive drug delivery system. Losartan potassium was prepared by direct compression method by using various polymers such as HPMC K4M, xanthan gum and Carbopol 971p at various concentrations. Sodium bicarbonate and sodium alginate were incorporated as gas generating agents and foaming agents. Lubricating agent like talc and magnesium stearate, lactose as sweetening agent, and microcrystalline cellulose as binding agent, FTIR spectroscopy study reveals that no interaction between drug and polymers. F1-F12 formulations were developed, and evaluated for thickness, weight variation, friability, drug content, floating time, lag time and in-vitro drug release. The in-vitro cumulative % drug release of all formulation ranged from 94.28% to 98.88% 0at 12hr. the floating time and lag time for the optimized formulation F9 was found to be 20min and 12hrs respectively. In-vitro drug released was found to be 98.88% for F9 at the end of 12hrs and which was considered as best formulation
Ray et al., (2010) was developed an optimal gastro retentive drug delivery system (GRDDS) for administering Losartan. Additionally, the influence of optimized GRDDS on the bioavailability of Losartan and the formation extent of active metabolite E3174 by CYP2C9 polymorphism was investigated. Swellable and floatable GRDDS tablets combining hydroxyethyl cellulose (HEC), sodium carboxymethyl cellulose (NaCMC), and sodium bicarbonate were prepared at various compression pressures for evaluating swelling characteristics and floating capacity.
Londhe et al., (2010) was developed Verapamil hydrochloride bi-layer floating tablets have two layers one immediate release layer and second floating sustained release layer. Verapamil hydrochloride bi-layer floating tablet releases drug in two phase’s i.e. immediate and sustained drug release. Direct compression method was used to formulate bi-layer floating tablets. All bi-layer formulation float more than 12 h and sustained drug release above 12 h.
Mina et al., (2009) was developed a gastro retentive controlled release drug delivery system with swelling, floating, and adhesive properties. Ten tablet formulations were designed using hydroxypropylmethylcellulose (HPMC K15M) and/or sodium alginate (Na alginate) as release-retarding polymer(s) and sodium bicarbonate (NaHCO3) or calcium carbonate (CaCO3) as a gas former. Swelling ability, floating behavior, adhesion period and drug release studies were conducted in 0.1 N HCl (pH 1.2) at 37 ± 0.5 C. The tablets showed acceptable physicochemical properties.
Annand et al., (2009) novel gastro retentive controlled release drug delivery system of verapamil HCL was formulated in an effort to increase the gastric retention time of the dosage form and to control drug release. Buoyancy was achieved by adding an effervescent mixture of sodium bicarbonate and anhydrous citric acid. In vitro drug release studies were performed, and drug release kinetics was evaluated using the linear regression method.
Chen et al., (2010) investigated the effect on compression pressure using appropriate ratio of hydroxy ethyl cellulose to sodium carboxymethyl cellulose (NaCMC), and sodium bicarbonate on losartan tablets. He concluded that compression at low pressure resulted in tablet floating over SGF for more than 16 h and swelling to 2 cm in diameter within 3 h.
Objectives
To fulfill the aim of investigation, following objectives were followed step-by-step in order to complete the task of work:
Selection of dose of drug.
Selection of pore creating substance.
Selection of channel former (release modifier) and its effect on drug release.
Effect of post compression curing on drug release, hardness, friability and floating behavior.
Preparation of preliminary batches of tablet containing losartan potassium.
Characterization of preliminary batches of prepared tablet containing losartan potassium.
Preparation of optimization batches of tablet containing losartan potassium.
Characterization of optimization batches of prepared tablet containing losartan potassium.
Materials and Methods
Materials
Losartan Potassium Was Procured From IPCA Laboratory, Silvassa, Polyethylene Oxide And Camphor Were Procured From Astron Chemicals Ahmedabad. HPMC Are Obtained From NR Chemicals Limited, Mumbai. Magnesium Stearate And Talc Were Procured From Chemdyes Corporation, Rajkot, Microcrystalline Cellulose Powder Are Obtained From Microwax Private Limited, Ahmedabad. The Residual Reagents Were Used Having Analytical Grade.
Methods
Direct compression method was selected to manufacture of gastroretentive floating tablet of losartan potassium tablets. Losartan potassium and excipients ware weighed for practical yield and the some was recorded.
The ingredient was weigh accurately and mixed thoroughly in increasing order of weight for 10 min. batches were compressed on Rotatory tablet compression machine using 9.5 mm flat punch. Density of tablets was kept constant 1.0 g/cm3. For curing experiments tablets of all batches were stored in an oven at 50 ℃ for 24 hours.
Methodology
Preformulation study
Preformulation study of dosage form is the first and foremost step in developmental process of any drug substance. Preformulation studies are defined as a study of physical and chemical phenomenon of drug itself and with any excipient.
Angle of repose
The angle of repose has been set at the maximum angle possible between the surfaces of a pile of powder and horizontal plane. Here the fixed funnel method was employed. A funnel was set with its crest at a given height (h), above a level horizontal surface on which a graph paper was placed. The powder was watchfully poured through a funnel till the apex of the conical pile just touches the top of the funnel. By using the below-given formula, the angle of repose was calculated.
Where,
θ = angle of repose,
h = height of pile,
Bulk density
It is the ratio of the mass of the powder taken with its bulk volume. The shape and cohesiveness of particles, particle size distribution, was depended on bulk density. A fixed quantity of powder was cautiously poured into the graduated measuring cylinder through an appropriate size funnel and volume was calculated, and it is called as bulk volume. Bulk density is expressed in kg/m3and is given by
Where,
ρb= bulk density (kg/m3),
M = mass of powder (kg),
Vo= bulk volume of powder (m3).
Tapped density
In a clean, dry 100 ml measuring cylinder 10 g of powder was loaded. The loaded cylinder was then tapped 100 times by tapped density apparatus or manually by hand from a fixed height and then tapped volume, tapped density was measured.
Which is specified in kg/m3and calculated by following formula
Where,
ρt= tapped density (kg/m3),
M = mass of powder (kg),
Vt= tapped volume of powder (m3).
Compressibility index
From the values of bulk and tapped densities a secondary method for determining powder flow is Carr’s index. The powder arch potency or bridge strength and stability of the powder were directly measured by the percentage compressibility. Each formulation’s Carr’s index was calculated by the below-given equation
Carr’s index= [(ρt –ρb) /ρt] x 100
Where,
ρt= tapped density (kg/m3),
ρb= bulk density (kg/m3)
Post-compression evaluation parameters:
Tablet shape
Shape is checked by magnifying lens after compression. It mainly includes shape and size of tablet. Also, it affects texture, any embossing matter, Color.
Tablet dimensions
In this three tablets are randomly taken and then their thickness and diameter are measured by Vernier caliper or by using calibrated screw gauze.
Weight variation test
Twenty tablets are selected and weighed individually. The the average weight and standard deviation is calculated. Test passes when not more than two tablets deviate from average weight.
Hardness
Expressed in kg/cm2 and it is checked using Monsanto hardness tester by randomly picking three tablets. Hardness helps in knowing ability of the tablet to withstand mechanical shock during handling of tablets
Friability
20 tablets are taken randomly and placed on a sieve. Loose dust is removed with the aid of air pressure or a soft brush. Tablet samples are weighed accurately and placed in Friabilator (Veego VFT-DV). After the given number of rotations (100 rotations) loose dust is removed from the tablets as before. Finally, tablets are weighed. The loss in weight indicates the ability of the tablets to withstand this type of wear. The percent friability is determined by using following formula,
% Friability= [(Initial weight - Final weight) / Initial weight] x 100
If % friability of tablets is less than 1% is considered acceptable.
Tablet Density
It is an important parameter in case of floating tablets. If density is less than gastric fluid (1.004) than only the tablets will float.
It is calculated using formula:
V=πr2h
D=m/v
Where,
r =Radius of tablet
h=crown thickness (g/cc)
m=Mass of tablet
FTIR Study
The possible chemical interactions of losartan Potassium with formulation polymer Polyethylene Oxide and other excipients were examined by FTIR spectroscopy (Bruker, Alpha-E, Germany) using KBr press pellet technique. Few milligram of sample mix with 100 time of potassium bromide and pressed by polymer press (PFM15, Techno search Instrument) by applying 5-ton pressure for 5 min. This pellet was placed on instrument. FTIR spectral scans were recorded in the range between 4000-600cm-1
Optimization of gastroretentive floating tablet of losartan potassium using 32 full factorial design
Based on the results of preliminary investigations of experimental work of Floating tablet to optimize the effect of variables on desired responses by keeping fixed concentration of other variables optimization was carried out in further investigation.
This can be easily analyzed and understood using established statistical design of experiment tools such as factorial designs are considered the most effective in estimating the influence of individual process with minimum experimentation and time, where all factors are studied in all possible combinations. Based on the factorial design of experiment, the optimization technique encompasses the generation of model equations for the investigated responses over the experimental design to determine optimum formulation. Full factorial (32) design was carried out in further investigation as shown in Table.
Table 1
Full factorial design layout for floating tablets of losartan potassium
Table 0
Assay of tablet
The Assay of Floating tablet ranged from 92.28±0.004% to 99.03±0.003%. They were well within the pharmacopoeia standard limits of 90% – 110%.
In-vitro dissolution study
Dissolution study is performed using USP paddle apparatus by maintaining optimum temperature i.e. 37 ℃ at 50 rpm rotational speed. At various time interval 5ml sample is withdrawn and is replaced with same amount of buffer.
In vitro buoyancy studies
The in-vitro buoyancy was performed in USP Apparatus 2 (Paddle) method (Electro lab, Mumbai TDT-08L) in 0.1 N HCl, pH 1.2 (900ml) maintained at 37±0.5℃ at 50 rpm and the time required for the tablet to rise to the surface and float was determined as floating lag time (FLT) and total floating time (FT) was also determined.
Stability study
Stability is the essential factor for quality, efficacy and safety of drug product. The drug product with insufficient stability can result in change of their physical (hardness, dissolution rate,) as well as chemical characteristics (formation of high risk and decomposition substances).
Effect of pH on floating behavior
Floating behavior of tablets was studied in 0.1 N HCl, pH 1.2, Distilled water and each 100 ml used to study the effect of pH on floating behavior.
I Took 6 Trials Batches F1to F6 In F5 Batch Is Better Results So I Optimized This Final Batch Over F5 Batch In Factorial Design.Batch F5 contain 90 0f PEO and 40 mg Camphor Shows 92.79% of drug release after dissolution from Floating tablets.
Results
Table 2
BD, TD, %CI, HR and Angle of repose for losartan potassium
In the FTIR spectrum of floating tablet containing Losartan Potassium and other excipient, various characteristic peaks of Losartan Potassium and Polyethylene Oxide, Camphor are appeared without any significant shifting of peaks. In short, floating tablet showed significant characters of Losartan Potassium in the FTIR spectrum, suggesting, no interaction between drug and excipients, used in the formulation.
Table 3
Formulation composition foroptimization batches with variables
Table 4
Results of in-vitro evaluation study of losartan potassium floating tablets of batches ‘LS1 to LS9 ’
Table 5
Results of In-vitro dissolution release of losartan potassium floating tablets of batches ‘LS1 to LS9’ in 0.1 N HCl, pH 1.2
Figure 4
Comparative in vitro dissolution profile of losartan potassium from floating tablets of batches LS1-LS9 in 0.1 NHCl, pH 1.2
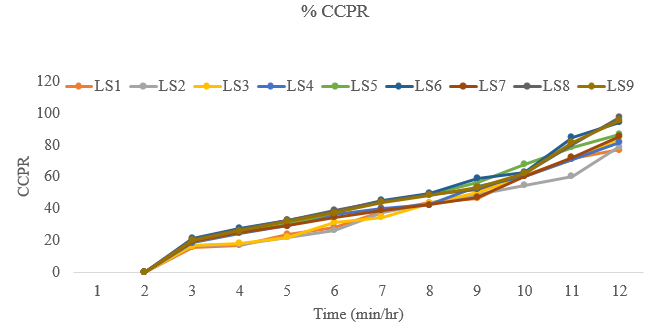
Table 6
Results of in-vitro buoyancy study of losartan potassium floating tablets of batches ‘LS1 to LS9’ in 0.1 N HCl, pH 1.2
Figure 5
Image showing buoyancy of losartan floating tablets of batch LS6 performed in 0.1 N HCl, pH 1.2 measured at ‘0, 6 and 12 hr.’

Table 7
Stability study:
Results of Stability study of optimized Losartan tablets floating tablets batch ‘LS6’
Conclusion
From the results of preliminary batches of floating Tablet containing Losartan Potassium, it was found that amount of Camphor and Polyethylene oxide affecting on the responses of floating formulation. Hardness, floating time, and drug release were dependent on the amount incorporated camphor. Minimum 40mg of camphor and 90 mg of PEO were necessary for floating of tablets up to 12 hrs. Post compression curing increased the hardness and floating time of tablets.
Purpose of optimization study was to get suitable amount of Camphor and PEO to attain maximum amount of drug release from floating tablet matrix as well as sufficient hardness, floating time. All FT demonstrated an initial burst release of immediate release layer within 15 min was due to Camphor. Formulation LS6 containing 40 mg of Camphor and 90 mg of PEO was determined to be the optimized formula which possess satisfactory quality parameters both in process and parameter of finished product. It shows floating time of more than 12 hours, cumulative % drug release of 27.37 % at the end of 30 minutes, cumulative % drug release of 58.93 % at the end of 6 hrs, and cumulative % drug release of 93.97 % at the end of 12 hr.
The stability studies were conducted for 15 Days at two conditions i.e. at ambient temperature (27±2 C; 65±5 % RH) and at higher temperature storage condition (40±2 C; 75±5 % RH) for tablet of optimized batch LS3 exhibited no any deleterious change in physical appearance, hardness, and drug content and dissolution profile.
Thus, results of the current study clearly indicate, a promising Potential of the Losartan Potassium Floating tablets as an alternative to the conventional dosage form, which give sustain release of drug from floating tablet of drug up to 12 h from matrix tablet, eliminate the need of bid dosing and also increase the bioavailability of Losartan Potassium.
It was concluded that sublimation method can be utilized for desired floating tablet formulation of losartan potassium at laboratory scale using camphor as sublimating agent, HPMC K4 as a dry binder and PEO as a release retarding agent.



