Introduction
Cyclobenzaprine Hydrochloride (CBZ) chemically known as 3-(5H-Dibenzo[a,d]1 annulen-5-ylidene)-N,N-dimethyl-1-propanamine hydrochloride.2 (Figure 1) The molecular formula & molecular weight of Cyclobenzaprine isC20H21N and 275.4g/mol. Cyclobenzaprine is a off-white crystalline tricyclic amine salt that is freely soluble in water and alcohol, slightly soluble in chloroform and methylene chloride, practically insoluble in hydrocarbons. Cyclobenzaprine is a centrally acting skeletal muscle relaxant with antidepressant activity.3, 4 The exact mechanism of action of cyclobenzaprine has not been fully determined, but this drug seems to primarily act at the brain stem to reduce tonic somatic motor activity, influencing both gamma and alpha motor neurons leading to a reduction in muscle spasms. cyclobenzaprine is used short-term to treat muscle spasms associated with acute, painful musculoskeletal conditions. It is usually used along with rest and physical therapy. It works by helping to relax the muscles.5, 6
Literature survey revealed that many analytical methods like UV, HPLC, HPTLC DSC, FT-IR and LC-MS for the determination of cyclobenzaprine in pure drug, pharmaceutical dosage forms and in biological samples have been reported.7, 8 But no method have been reported for dissolution study of cyclobenzaprine hcl in solid dosage forms, So, the aim of the existing work to development of dissolution test method using RP-HPLC method for analysis of cyclobenzaprine HCL in Tablets and to validate dissolution method accordance with ICH guidelines for, precision, linearity and range, accuracy, ruggedness and robustness study.
Materials and Methods
Chemicals and reagents
Cyclobenzaprine HCL was kindly obtained as a gift sample from Zim Laboratories Ltd. (Nagpur). Commercial tablets containing cyclobenzaprine hydrochloride 5mg was procured from the local chemist shop manufactured by Macleods Pharmaceuticals Ltd. Acetonitrile and Methanol used was of HPLC grade. Double distilled water was used for preparing dissolution media and HPLC mobile phase. 0.1 N HCl and Phosphate Buffer pH 6.8 was prepared as per the pharmacopoeia. Other chemicals include potassium dihydrogen phosphate; ortho phosphoric acid and hydrochloric acid of GR grade were used. 0.1 N HCl, acetate buffer pH 4.0, Phosphate Buffer pH 6.8 was prepared as per the pharmacopoeia.
Instrumentation
Uv-Spectrophotometer: Jasco-V-630, HPLC: Analytical Technology Limited, Analytical HPLC isocratic system consisted of Shodex C18-4E (5µm) 250×4.6mm, Uv-3000 detector and P-3000 Pump, Rheodyne injector with 20μL capacity, Dissolution Apparatus: Electrolab Tablet Dissolution tester-TDT-06P, Weighing balance: Shimadzu AUX 220, pH-meter: Digital pH Meter (model 111E), Sonicator: PCI Mumbai 3.5L100H.
Determination of working wavelength
The standard solutions cyclobenzaprine HCl was prepared in Methanol having concentration 10 µg/ml and subjected to U.V. spectrophotometeric study to determine λ max of drug over the wavelength range 200-400 against Methanol as blank solution. Cyclobenzaprine shows maximum absorption at 223.6 and 289.4nm, the wavelength selected was 289.4 nm. The spectrum recorded is shown in Figure 2.
Preparation of phosphate buffer (pH 3.0)
Dissolve 1.36 g of potassium dihydrogen orthophosphate and 2 mL of triethylamine in 800 mL of water, Adjusted pH to 3.0 with orthophosphoric acid and add sufficient water to produce 1000 mL.
Preparation of mobile phase
A mixture of Acetonitrile and Phosphate Buffer (pH 3.0) in the ratio of 65:35v/v was prepared. The mobile phase was sonicated for 15 min and filtered through nylon membrane filter (0.45μm).
Preparation of standard stock solution
A standard stock solution having concentration 1000 µg/mL of CBZ was prepared in mobile phase.
Preparation of working standard solution
A 1.0 mL of the above standard solution was diluted upto 10.0 mL to prepare a solution having concentration 100 µg/mL of CBZ, from this solution further 2.0 mL was pipetted and diluted upto 10.0 mL to get working standard solution having concentration 20 µg/mL of CBZ.
Chromatographic conditions
Chromatography was achieved on a Shodex C18-4E column(250 x 4.6 mm). The mobile phase was a mixture of ACN: Phosphate buffer in the proportion of 65:35 % v/v and adjusted pH to 3.0 with orthophosphoric acid which was filtered (0.45μm) and degassed before use. All analysis was performed at room temperature at a flow rate of 1 mL/min. Detection was made at 289.4 nm. A 20 μL volume injection were utilized for analysis.
System suitability parameters
The system suitability study was carried out using six replicates injections of standard solution containing 20µg/mL of CBZ was prepared in mobile phase. A 20µL of solution were injected through manual injector and analyzing the chromatograms for peak area, theoretical plates, % RSD and tailing factor. Results are shown in Table 1.
Dissolution Test Conditions
Drug dissolution tests were carried out with USP apparatus II (paddle type) at 50 rpm with dissolution volume of 900mL. Thermostatic bath was used to maintain the temperature of the cell at 37°C±0.5°C. Various dissolution media’s were tried out of which 0.1 N HCL (1.2 pH) was selected. Weighed and dropped 1 tablet in each of the six dissolution vessel containing 0.1 N HCL (pH 1.2) for the drug under analysis. Aliquots of 5.0 mL were withdrawn at 5, 10, 20, 30, 40, 50 and 60 min time interval, used as sample and replaced with an equal volume of the fresh medium to maintain a constant total volume. After the end of each time point, sample aliquots were filtered and chromatographed. The percentage drug release was estimated by validated HPLC method at each time point.
Table 1
Results of system suitability parameters
Table 2
Results showing effect of change in USP apparatus on CBZ
Table 3
Results showing effect of change in volume of dissolution media on CBZ
Table 4
Results showing effect of change in dissolution media (buffer)on CBZ
Table 5
Final dissolution method parameters for the drug
|
Drug |
Dissolution media |
Media volume |
USP Apparatus |
RPM |
|
Cyclobenzaprine |
0.1N HCL |
900mL |
Type-II (Paddle) |
50 |
Table 6
% Release of drug under final selected chromatographic condition
Table 7
Observation of linearity response of CBZ
|
S.No. |
Concentration (µg/ml) |
A.U.C (mV) |
|
1 |
2 |
284272 |
|
2 |
4 |
463967 |
|
3 |
6 |
637275 |
|
4 |
8 |
793641 |
|
5 |
10 |
987361 |
|
Correlation coefficient R2 |
0.9991 |
|
Table 8
Observations and result of recovery for CBZ
Table 9
Results of repeatability precision study
|
Sample |
% Dissolution of CBZ at 15 min |
|
1 |
100.55 |
|
2 |
99.04 |
|
3 |
99.85 |
|
4 |
100.53 |
|
5 |
99.16 |
|
6 |
99.29 |
|
Mean |
99.74 |
|
%RSD |
0.68 |
Table 10
Results of intra-day precision study
|
Time interval (h) |
A.U.C(mV) |
% Dissoution |
|
1 |
590412 |
100.54 |
|
582496 |
99.20 |
|
|
2 |
584712 |
99.57 |
|
593214 |
101.02 |
|
|
3 |
573122 |
97.60 |
|
571004 |
97.24 |
|
|
Overall mean |
98.83 |
|
|
%RSD |
1.25 |
|
Table 11
Results of inter-day precision study
|
Time interval |
A.U.C(mV) |
% Dissoution |
|
1st day |
574522 |
97.84 |
|
574414 |
97.78 |
|
|
2nd day |
571026 |
97.24 |
|
563201 |
95.91 |
|
|
3rd day |
572287 |
95.75 |
|
561057 |
95.55 |
|
|
Overall mean |
96.41 |
|
|
%RSD |
0.95 |
|
Table 12
Results of ruggedness study
|
Analysts |
% Dissolution |
Overall Mean |
%RSD |
|
Analyst-I |
100.83 |
100.07 |
1.08 |
|
Analyst-II |
99.31 |
Table 13
Results for robustness study
Dissolution method parameter optimization for CBZ
Various dissolutions were performed to optimize the parameters like dissolution media, dissolution media volume, apparatus and rpm, using the optimized chromatographic conditions and the solubility data of the drugs to select a set of parameter that will give maximum % release of the drug.
Study of change in USP apparatus type
To study the effect of change in USP apparatus, 0.1 N HCL (pH 1.2) was selected as dissolution media. A media volume of 900 mL and thermostatic bath temperature was kept constant and the dissolution was performed on two different USP apparatus. The results were evaluated for cyclobenzaprine. The % drug release for two apparatus types were calculated.
Method validation
The dissolution test method was validated to through the determination of linearity, precision, accuracy, solution stability. Prior to injecting sample solutions, the column was equilibrated for at least 30 min with the mobile phase flowing through the system.
Linearity
The linearity for CBZ with respect to concentration was demonstrated by considering the label claim of the CBZ as 100% target concentration. Linearity was studied by analyzing five standard solutions covering the range of 2-10µg/ml. From the working standard solution (100 µg/ml) 0.2ml, 0.4ml, 0.6ml, 0.8ml and 1.0ml of aliquots were pipetted out into a series of 10 mL volumetric flasks and volume made up to the mark with the mobile phase to get concentrations in the range of 2µg/ml-10µg/ml respectively. Calibration curve of peak area versus % target concentration was plotted by injecting the above prepared solutions and calibration curve is shown in Figure 3.
Accuracy
The accuracy of the proposed method was evaluated by spiking method i.e. adding known amount of CBZ standard drug (80%-120%) to that of target dissolution concentration as per the labeled claim of 5 mg CBZ formulation respectively. Accordingly pure drug was added at the selected levels along with 5.0 mg tablet. Dissolution of the drug was performed using optimized dissolution parameters. Aliquots of 1.0 mL were withdrawn and filtered through whattman filter paper and analyzed by chromatographic method at spiked concentration levels of 80%, 100 and 120%, respectively. Each concentration was analyzed in triplicate. The system was allowed to equilibrate for 30 minutes by passing mobile phase through chromatographic column at a flow rate of 1.0 mL/min. After equilibration, triplicate injections of test solution of different concentration having a volume of 20µL were injected. Total amount of drug estimated and recovered was calculated.
Precision
The precision of the method was evaluated by measuring the precision expressed as % RSD. Tablet samples were subjected to dissolution test condition 900 mL of dissolution medium (0.1 N HCL buffer) pre-heated at 37°C±0.5°C, paddle with stirring rate of 50 rpm). The test sample were obtained by performing the dissolution of the drug under analysis using optimized dissolution parameters and were chromatographed by using finalised chromatographic parameters. The study was performed with 3 consecutive studies i.e. Repeatability precision study, Intraday Precision study and Inter-day precision study. The obtained test samples were injected at 1st h, 2nd h and 3rd h. Chromatograms were recorded for Intra-day precision study. Similarly, the same sample solution was injected on 1st, 2nd, and 3rd day for Inter-day precision study. The chromatograms so recorded and result were calculated.
Range
Range of the Analytical procedure is the interval between the upper and the lower concentration (amounts) of analyze in the sample for which it has been demonstrated that the analytical procedure has a suitable level of precision, accuracy and Linearity.
Ruggedness
The ruggedness of an analytical method is the degree of reproducibility of test results obtained by the analysis of the samples under a variety of conditions. The method was studied for ruggedness by analysing sample and standard preparation by two different analyst.
Robustness
The robustness of an analytical procedure is a measure of its ability to remain unaffected by small, but deliberate variations in method parameters and provides an indication of its reliability during normal usage. The robustness of test method was carried out for following parameters:
a) Change in flow rate, b) Change in pH of mobile phase, c) Change in detection wavelength
Results and Discussion
HPLC Method development and dissolution method validation
The optimized chromatographic condition mentioned below was kept constant throughout the experimentation and mobile phase was allowed to equilibrate with stationary phase which was indicated by a steady line.
System: Analytical HPLC
Stationary Phase: Shodex C18-4E (250 x 4.6mm, 5µm)
Mobile phase: ACN: Phosphate buffer (pH 3.0) (65:35 % v/v)
Detection wavelength: 289.4nm
Column tempreature: 25°C-30°C
Flow rate: 1.0 mL/min
pH: 3.0
Injection volume: 20 µL
Run time: 9 min
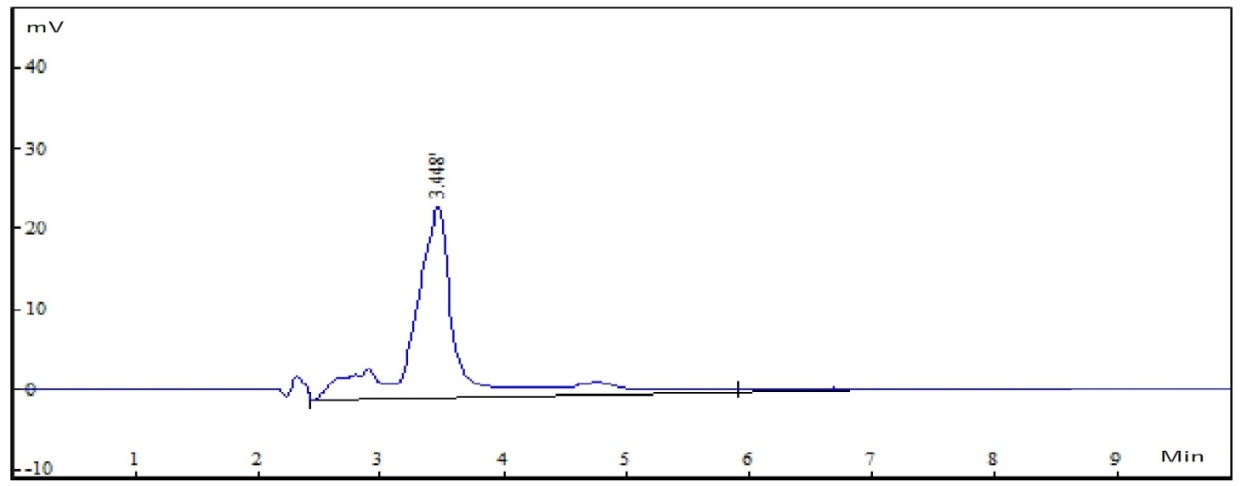
A 20 μL solution of above mix standard was injected through manual injector and chromatogram was recorded. A standard chromatogram for cyclobenzaprine HCl is shown in Figure 4.
Optimization of dissolution method parameters for estimation of cyclobenzaprine
Various dissolutions were performed to optimize the parameters like dissolution media, dissolution media volume, apparatus and rpm, using the optimized chromatographic conditions and the solubility data of the drugs to select a set of parameter that will give maximum % release of the drug. The chromatograms of dissolution analysis of formulation under study at selected intervals recorded under optimized chromatographic parameters are shown in Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10, Figure 11.
Change in USP apparatus
The result of % release of drug is shown in Table 2. From the table, it was observed that the release of drug in USP I was slow as compared to USP II. Also the release of drug at 50 rpm was found to be optimum as compared to other rpm conditions; therefore USP II and 50 rpm were selected as one of the optimized dissolution parameter and were further used in the experimentation.
Change in the volume of dissolution media
The result of % release of drug is shown in Table 3. From the table, it can be observed that the percent drug release in a media volume using 500 mL was found to be less as compared to a media volume of 900 mL. Hence media volume of 900 mL was selected as one of the optimized dissolution parameter and was further used in the experimentation.
Change in dissolution media (Buffer)
The result of % release of drug is shown in Table 4. From the Table, it was observed that the release rate of the drug obtained was less in Phosphate Buffer (pH 6.8) as compared to 0.1 N HCl at Q point 15 min therefore, 0.1N HCl was selected as optimized dissolution media and further used in the experimentation. The finalized dissolution parameter selected for the dissolution test method of cyclobenzaprine are shown in Table 5 and percent release of drug under final chromatographic and final dissolution parameters are shown in Table 6 for cyclobenzaprine.
Validation parameters
The peak area of linearity solutions noted was plotted against the corresponding concentrations to obtain the calibration graph. The correlation coefficient were found to be 0.9991 and the calibration data is presented in Table 7. The proposed method gives linear results with wide range of concentration of drug.
The results of accuracy data at different spike level are shown in Table 8. From the accuracy studies the mean % recovery of drugs at each accuracy level was found to be 100.04%, Which is found to be in the acceptance range of 95%-105%. It was proved that method is accurate.
The Precision of the dissolution study carried out as repeatability, intra-day and inter-day variations. The % release was found to be in acceptance level and % RSD of drugs from repeatability study was found to be 1.68, the % RSD for Intra-day study was found to be 1.25 and the % RSD for Inter-day study was found to be 0.95 respectively ascertaining the precision of method. The observation and the result of precision study for drug is summarized in the Table 9, Table 10, Table 11.
The observation and the result of ruggedness study for drug is summarized in the Table 12. Ruggedness of the method is evaluated by analysis of aliquots from homogenous slot by two different analyst. The RSD between two different analysts should not be more than 2.0%. The proposed method was found to be rugged.
The robustness of the method was evaluated by injection of the sample at deliberately varying the chromatographic conditions i.e change in flow rate by 0.2mL/min, change in wavelength and pH by ±5. The method was found to be robust. The results obtained by robustness study are given in? Table 13.