Introduction
The infectious disease, severe acute respiratory syndrome (SARS) is caused by a newly identified human corona virus (SARS-CoV). No effective drug existed to treat SARS-CoV infection as of now.1
Positive sense RNA viruses ranging from 60 nm to 140 nm diameter with spike like projections on its surface revealing crown like appearance are enveloped in Corona viruses. They are visible and identified only under the electron microscope and due to crown like appearance the name coronavirus. HKU1, NL63, 229E and OC43 are types of four corona viruses mainly found in circulation in humans, and generally cause mild respiratory disease.
The origin of spread of disease is in Wuhan City of Hubei Province of China to the rest of the world. The 2019 novel corona virus (2019-nCoV) as it is now called, is rapidly spreading and now several cases of corona virus disease 2019 (COVID-19) and so many deaths have been reported. Fortunately, so far, children have been infrequently not succumbed to death. But the future course of this virus is unknown.2
The efforts are being made to reduce transmission via standard public health interventions based on isolation of cases and tracing of contacts, as the corona virus disease spreads. It is predicted that this strategy could contribute to reducing the overall size of an outbreak, but it will still be insufficient to achieve spread and outbreak control of COVID-19 when the basic reproduction number (R0) is higher than 1·5 or the proportion of contacts traced is lower than 80%.3
The past two decades witnessed two events wherein crossover of animal beta CoVs to humans has resulted in severe disease. The first was in 2002–2003 while a new CoV of the β genera in bats crossed over to humans via the intermediary host of palm civet cats in the Guangdong province of China. This virus, designated as SARS-CoV. The second was in 2012 in the Middle East respiratory syndrome-CoV (MERS-CoV), of bat origin, obtained in Saudi Arabia with dromedary camels as the intermediate host and affected so many people with high mortality rate.4
The onset of pneumonia and acute respiratory infection are serious illness observed during initial stage.
The symptoms of COVID-19 include fever or chills, Cough, difficulty in breathing, fatigue, muscle or body pain, headache, loss of taste or smell, diarrhea, Sore throat, Congestion or runny nose, Nausea and vomiting,
Transmission
The virus is thought to spread from person-to-person via droplet transmission (large respiratory droplets that people sneeze, cough or drip), aerosol transmission (when someone coughs or sneezes in the room, aerosolized droplets, from talking and singing), contact transmission (touching a contaminated surface then touching your mouth, nose or eyes), direct transmission (kissing, shaking hands etc.)
Prevention
Prevention is better than to cure any disease or any infection. It is advised to wash hands regularly and thoroughly with soap and water (lather for 20 seconds) or use an alcohol based (at least 60%) hand sanitizer.
Some additional measures to prevent the spread of COVID-19
Avoid contact with others who are sick
Avoid touching mouth, nose, eyes or face
Cover coughs and sneezes (into a tissue or into elbow)
Clean and disinfect surfaces (alcohol or bleach based cleaning solutions work best for Coronaviruses).
Face masks will not protect from COVID-19 directly, but can help reduce your risk of exposure to the virus, remind you to avoid touching your face, and will help prevent the spread of the disease to others.
Recommended social distancing.
Self isolation when not feeling well.
Healthy Vitamin D Levels5
Till now, there is no Food and Drug Administration-approved drugs for the treatment ofCOVID-19. The standardized clinical trial data are needed to identify safe and effective treatments for COVID-19. As in the management of any disease, methodology of treatment ultimately based on the condition of patient and their health care provider.6
Considering the facts some promising and significant drugs of this category discussed here.
Reverse transcription inhibitors
The strategy to combat SARS-CoV-2 infection involves targeting the reverse transcription step by blocking RdRp and thus preventing viral replication. A few possible inhibitors are nucleoside reverse-transcriptase inhibitors (NRTIs), nucleotide reverse-transcriptase inhibitors (NtRTIs), non-nucleoside reverse-transcriptaseinhibitors (NNRTIs) and nucleoside reverse transcriptase translocation inhibitors (NRTTIs). Remdesivir is effective against many other viruses, and medical experts are hoping to have similar compounds useful in combating pathogen liable for COVID-19.
The mistake generally made lately is to think that any antiviral would be effective against [the coronavirus]. Remdesivir is a promising antiviral being tested for the corona virus, experimented and developed to treat Ebola and also proved to be effective against the SARS and MERS viruses in vitro, could be effective.7
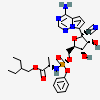
IUPAC name
2-ethylbutyl (2S)-2-[[[(2R, 3S, 4R, 5R)-5-(4-aminopyrrolo [2,1-f]1, 2, 4 triazin-7-yl)-5-cyano-3, 4-dihydroxyoxolan-2-yl] methoxy-phenoxyphosphoryl] amino]propanoate.8
Remdesivir is a mono phosphoramide prodrug. Its molecular mass is 602.6 g/mol and chemical formula is C27H35N6O8P. Remdesivir is a nucleotide analog. Majority viruses are single stranded RNA viruses like corona viruses (including MERS-CoV and SARS-CoV-2) Lassa fever virus, Junin virus, Ebola-Marburg virus, respiratory syncytial virus, Nipah virus and Hendra virus. Remdesivir has a wide spectrum of antiviral properties against earlier single stranded RNA virus. It is studied, Remdesivir first enters the host cells, metabolize and capable of reducing RNA replication of SARS-CoV, MERS-CoV, zoonotic and endemic human delta coronaviruses under in vitro conditions, while in vivo results showed antiviral potential against bat and human corona viruses in primary epithelial and lung cell culture systems.
Remdesivir was proposed as a therapeutic agent against SARS-CoV-2., when studies found that remdesivir was effective against MERSCoV;it reduced the viral loads in the affected part in mice, therefore supported to regain the normal pulmonary functions. After 12 days of Remdesivir administration, swab of nasopharyngeal and oropharyngeal taken and it was found that the viral load reduced significantly. A combination of Remdesivir and Chloroquine, an anti-malarial drug, in vitro study showed effective inhibition of SARS-CoV-2 growth in Vero E6 cells.US, Norway and France are conducting clinical trials to assess the efficacy of remdesivir for COVID-19 Remdesivir has been used to treat COVID-19 cases in the USA and Singapore. In the USA remdesivir was given intravenously and first subject of COVID-19 recovered.9
It is postulated that using polymerase enzymes from the corona virus which causes MERS, found that the enzymes can incorporate Remdesivir, which resembles a RNA building block, into new RNA strands. Immediately after adding on Remdesivir, the enzyme stops growth of more RNA subunits. This in turn results in halting the genome replication.10
Initially there is no drug for the treatment of COVID-19. However Remdesivir was showing promising result. But inventory of Remdesivir was very low, so recommendations are to use firstly in hospitalized patients with COVID-19 who require supplemental oxygen but not the forced oxygen delivery, ventilation or extracorporeal membrane oxygenation (ECMO).
There are insufficient data on the optimal duration of remdesivir therapy for patients with COVID-19 who haven't shown clinical improvement after 5 days of therapy so some experts extend the full Remdesivir treatment duration about 10 days.6
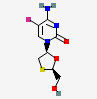
IUPAC name
5-fluoro-2-oxo-1H-pyrazine-3-carboxamide, Favipiravir possess antiviral activity. Favipiravir was initially developed to treat influenza in Japan.Corona outbreak in February 2020, in China and several other countries started favipiravir as an experimental treatment of COVID-19.11 Various Literatures reported that favipiravir acts as a broad-spectrum medication that prevents the multiplication of flavivirus, filovirus, poliovirus, arena viruses, and rhinovirus. The medication has recently been accounted in some studies as valuable in shortening the time of clinical recovery for COVID-19 patients. The use of favipiravir in the treatment of the viral disease was on temporary basis earlier. Some doctors and scientists also suggest using favipiravir even though don’t have sufficient good randomized clinical trials.There are also some partial satisfaction results of favipiravir. There must be proof of covid 19 either in the form of antigen test or RT-PCR test. The fever should be less than 7 days duration. Favipiravir should be used in early stage with quick diagnosis in selected patient, so Favipiravir must be stop if patients have vomiting and other side effects.
IUPAC name
1-β-D-ribofuranosyl-1H-1,2,4-triazole-3-carboxamide 12
Ribavirin is a synthetic nucleoside analogue (purine analogue). Ribavirin has antiviral activity against against SARS-CoV-2. FDA supported the therapeutic efficiency of Ribavirin, Remdesivir, Penciclovir, Favipiravir, Nitazoxanide, Nafamostat, and Chloroquine against this strain based on in vitro trials. Ribavirin works to hamper the function of polymerases, make it more difficult the RNA capping to destabilize the viral RNA and deliberately make difficult to replicate. At the same time, Ribavirin inhibits the function of inosine monophosphate dehydrogenase enzyme to prevent the production of guanosine and hence promotes degradation of viral RNA. It further lead to loss of virulence in progeny viruses. The ability of Ribavirin against SARS-CoV-2 is being tested in clinical trials in Hong Kong. According to the regulations for the prevention, diagnosis, and treatment of Novel Coronavirus give rise to pneumonia in China for temporary treatment of COVID-19, Ribavirin is one of recommended medications that is administered with a combination with either IFN alpha or Lopinavir-Ritonavir. Docking and modeling analysis using Ribavirin together with Sofosbuvir and Remdesivir indicated that Ribavirin is a influential medication for COVID-19 treatment and can be delivered either by intra-venous route or orally. At present Ribavirin and Sofosbuvirare are part of the therapeutic regimen to treat COVID-19 in some countries.9
IUPAC name
[[(2R)-1-(6-aminopurin-9-yl)propan-2-yl]oxymethyl-(propan-2yloxycarbonyloxymethoxy)phosphoryl]oxymethyl propan-2-yl carbonate;but-2-enedioic acid.
Tenofovir disoproxil fumarate is a nucleotide reverse transcriptase inhibitor. It is similar in structure either with Remdesivir or Ribavirin and therefore probably has antiviral activity against COVID-19. The patient with HIV on antiretroviral therapy shows lower rate of infection compared to individuals in the general population. Many patients who are on antiretroviral combination included Tenofovir disoproxil fumarate has low incidence of COVID-19.11
IUPAC name
4-amino-5-fluoro-1-[(2R,5S)-2-(hydroxymethyl)-1, 3-oxathiolan-5-yl]pyrimidin-2-one.8
Emtricitabine and tenofoviralafenamide are reverse transcriptase inhibitors. Their use in treatment of HIV and hepatitis B virus (HBV) already studied. Currently, only one trial combines Emtricitabine/Tenofovir-alafenamide and Lopinavir/Ritonavir to treat COVID19 patients.13
Table 1
Detailed report of commercially available Reverse transcriptase inhibitors in treatment of COVID-1914 (COVID-19 promising cure)
Other transcription inhibitors
Additionally FDA approved NtRTIs like adefovir, tenofoviralafenamide, tenofovirdisoproxil, abacavir, ganciclovir, and didanosine showed resemblance structural characteristics either with remdesivir or ribavirin, and so, probably have antiviral activity against SARS-CoV-2. Other transcriptase inhibitor like NRTIs (lamivudine, stavudine, zidovudine, emtricitabine, zalcitabine, and azvudine) and NNRTIs (efavirenz, nevirapine, delavirdine, and rilpivirine) may additionally have antiviral properties against SARS-CoV-2. Though a number of these drugs are evaluated in silico through molecular docking studies, further studies are warranted to determine their clinical efficiency.9
Conclusion
Although there is no antiviral drugs for COVID-19 so far, the use of some available antiviral drugs targeting specific steps within the life cycle of SARS-CoV-2 could be another therapeutic strategy for dealing with this pandemic. Fusion inhibitors, protease inhibitors and transcription inhibitors are some of the significant members of antiviral drugs to be considered. Apart from these antiviral drugs, several other effective methods are also being used to treat COVID-19 such as convalescent plasma, the use of which has shown a reduction in viral load and morbidity of patients. IFN-⍺/β group and IL- inhibitors have also showed promising effects and are currently being assessed in several clinical trials. Antiviral therapies are being further investigated for the treatment of COVID-19. These drugs inhibit viral entry (via the angiotensin-converting enzyme 2 [ACE-2] receptor and trans membrane serine protease-2, viral membrane fusion and endocytosis, As the viral replication may be particularly active early in the course of COVID-19, the therapy must have greatest impact before the illness progresses into the hyper inflammatory state which may characterize the next stages of disease, including critical illness. However care must be taken to use this class of drugs in medication as optimization is examined in every stages of infection with mild, moderate, severe, and critical illness with COVID-19.