Introduction
Trazodone HCl, 2-{3-[4-(3-chlorophenyl)-1-piperazinyl] propyl} - 1,2,4 - triazolo [4,3 - a] pyridin - 3 - (2H)-one mono hydrochloride, is a anti-depressant. Trazodone binds at 5-HT2 receptor. It acts as a serotonin agonist at high doses and a serotonin antagonist at low doses. Like Fluoxetine, trazodone's antidepressant activity likely results from blockage of serotonin reuptake by inhibiting serotonin reuptake pump at the presynaptic neuronal membrane. The sedative effect of trazodone is likely the result of alpha-adrenergic blocking action and modest histamine blockade at H1 receptor. It weakly blocks presynaptic alpha2-adrenergic receptors and strongly inhibits postsynaptic alpha1 receptors. Trazodone does not affect the reuptake of nor-epinephrine or dopamine within the CNS. In literature survey many analytical methods includes RP-HPLC1, 2, 3, 4, 5 UV-spectroscopic1, 2, 3, 4, 5 and HPTLC1 methods have been reported for the estimation of TZH in bulk, pharmaceutical formulation n and in biological samples. In present study, simple, economical, accurate reproducible analytical method with better detection range for estimation of TZH in its pure form and its pharmaceutical dosage were developed. This paper describes a UV-Spectrophotometric and Zero Order Derivative methods for estimation of TZH in bulk and pharmaceutical dosage form using Area Curve (AUC) technique. To our knowledge no method has been reported using water solvent on zero order UV-spectrometric method for estimation of TZH in Bulk and Pharmaceutical dosage form. Further methods validated as per ICH Q2R1 guideline.
Materials and Methods
Selection of common solvent
R.O. water was selected as common solvent for developing spectral characteristics of TZH. The selection was made after evaluating the solubility of TZH in different solvents ACN, MeOH,0.1N HCL
The stock standard solution was prepared by dissolving 10 mg of TZH in 100 mL of water to obtain a concentration of 100 µg/mL. The working standards were prepared by dilution of the stock standard solution.
Determination of λ max and linearity study
Taken portion 1.0 mL of TZH were transferred to 10 mL volumetric flask, diluted to mark with water to obtain concentration of 10 µg/mL for TZH. The resultant solution was scanned in UV range (200-400 nm) in 1.0 cm cell against solvent blank. The λmax of TZH was found to be 246 nm. In Method I absorbance at 246 nm was considered for analysis while for Method II two wave lengths 235.20 nm to 256.60 nm were selected for determination of Area Under Curve [AUC] (Figure 2).
Linearity curve
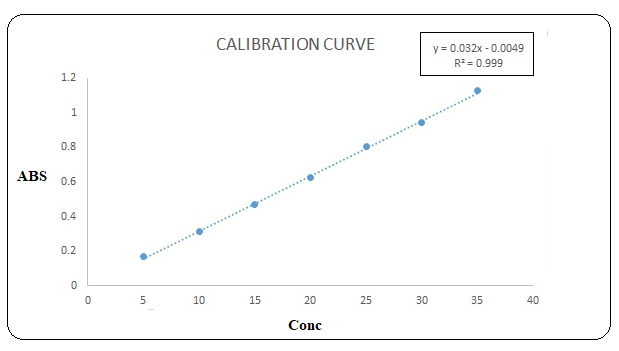
For linearity study, seven solutions of TZH of different concentrations (5-35 µg/ml) were prepared using stock standard solution, analysed by proposed methods and the obtained data were utilized to plot calibration curves results was reported inTable 2 for both methods.
Analysis of pharmaceutical formulation:=
An accurately measured weight of 20 tablet equivalent to 10mg of TZH was transfer into 100 mL volumetric flask and volume was made up to the mark with water, filtered through 0.45 µm Whatman filter paper. A suitable volume of solution was further diluted with water to obtain concentration 15 µg/mL of TZH. AUC were recorded in between the selected wavelengths and the concentrations were determined using respective linear regression equations. The analysis procedure was repeated for six times with same concentration.
Figure 2
Zero order spectrum of TZH showing lambda max and wave length selection between 235.20 and 256.60nm of AUC

Table 1
Analysis of Tablet (TZH)
|
|
Label Claim(mg) |
Conc (µg/ml) |
% Amount found [n=6] |
SD |
%RSD |
|
Method I |
100 |
15 |
99.41% |
0.004 |
0.49 |
|
Method II |
100 |
15 |
99.72% |
0.006 |
1.08 |
Table 2
Validation parameter
Method validation
The method was validated terms of accuracy, precision and ruggedness, sensitivity, repeatability.
Accuracy
The accuracy of tablet of TZH by both methods was evaluated through recovery experiments. To the pre-analyzed sample solutions of concentration 15µg/mL; a known amounts of stock standard solutions were added at different levels, that is, 80%, 100%, and 120%. The solutions were re-analyzed by the proposed methods. The experiments were performed for three times at each level for each method.
Precision (Intra-day and Inter-day precision)
Precision was determined as intra-day and inter-day variations.
Intra-day precision was determined by analysing the 10,15 and 20µg/mL of TZH solution for three times in the same day morning, afternoon, and evening. Inter-day precision was determined by analysing 10,15 and 20 µg/mL of TZH drug solution daily for three consecutive days over a period of week results are reported in Table 2 for bulk as well as pharmaceutical formulation by both methods.
Repeatability
Repeatability was determined by analysing 15 µg/mL of TRZ for six times and results reported in Table 2 for bulk and formulation by both methods.
Ruggedness
Ruggedness of the proposed method was determined by analysis of aliquots from homogenous slot by two analyst using same operational and environmental conditions and the results are reported in Table 2 for bulk and pharmaceutical formulation. by both methods.
Sensitivity
The sensitivity of proposed methods was determining in terms of Limit of Detection (LOD) and Limit of Quantification (LOQ) which were calculated using formulae “LOD = 3.3 ×N/B” and “LOQ = 10 ×N/B where N is average standard deviation of the absorbance or peak areas of the TZH and “B” is the slope of the corresponding calibration curve. LOD and LOQ was found to be 0.38 and 1.18 respectively for method I and LOD and LOQ was found to be 0.27 and 0.84 respectively for method II.
Result and Discussion
In water TZH obeyed linearity in the concentration range of 05- 35 µg/mL, a correlation coefficient (r2 > 0.999) and equation was found in Y= 0.032x-0.0049 for method I and a correlation coefficient (r2 > 0.995) and equation was found in Y= 0.1014x – 0.089 for method II Marketed brand of tablet form were analysed. The amounts of (TZH) determined by ‘Method I’ was found to be 100% and 99.5%, for bulk and pharmaceutical dosage form respectively; and The amounts of TZH determined by ‘Method II’ was found to be 99.76 and 98.43%, for tablet and parenteral dosage form respectively in both these methods precision was studied as repeatability (% RSD < 2) and inter and intra-day variations (%RSD < 2) for drugs. The accuracy of method was determined by calculating mean percentage recovery. It was determined at 80,100 and 120% level. The ruggedness of the methods was studied by two different analysts using the same operational and environmental conditions. The% recovery, Table 2.
Conclusion
The proposed method is rapid, accurate, precise and sensitive for the quantification of trazodone hydrochloride and its pharmaceutical dosage forms by the spectrophotometric method. The method based on the use of simple working procedure comparable to that achieved by sophisticated and expensive technique such as HPLC, and hence this method can be routinely used in quality control for analysis of trazodone hydrochloride in tablets.