- Visibility 395 Views
- Downloads 97 Downloads
- Permissions
- DOI 10.18231/j.ijpca.2024.049
-
CrossMark
- Citation
Development and validation of RP-HPLC method for simultaneous estimation of lidocaine and nifedipine in pure and combined dosage form
- Author Details:
-
Rupali Shinde
-
Chandni Chandarana *
Abstract
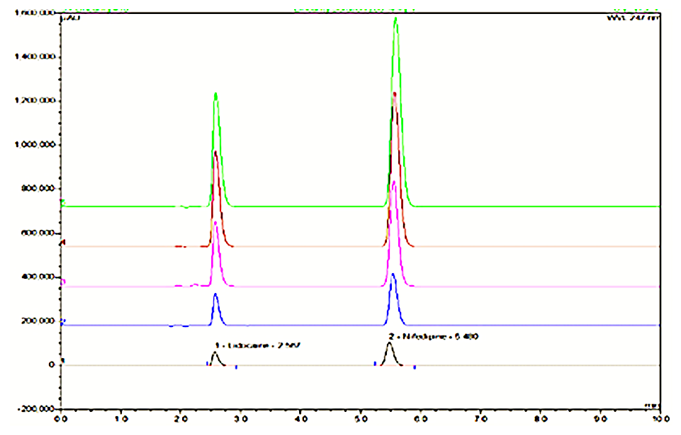
The simultaneous estimation of Lidocaine and Nifedipine using reversed-phase high-performance liquid chromatography (RP-HPLC) is essential for analysing both the bulk drug and its topical dosage form. The objective of this study was to develop a novel HPLC method for the simultaneous estimation of Lidocaine and Nifedipine. The method aimed to provide accurate and precise results for both the bulk drug and topical dosage form. The HPLC method employed a C18 column measuring (150 mm × 4.6 mm × 5 µm). A mobile phase consisting of a mixture of methanol and water in a ratio of 80:20 v/v was used. The flow rate was set at 1.0 ml/min, and the detection wavelength was set at λ-max 236 nm. The retention time for Lidocaine was determined to be 2.560 min, while for Nifedipine it was found to be 5.470 min. The developed HPLC method demonstrated good linearity for both Lidocaine and Nifedipine, as evidenced by the obtained correlation coefficients (R2) of 0.9890 and 0.9986, respectively. The accuracy studies yielded results within the acceptable range of 98.11% - 105%. Additionally, the precision of the method was established with % RSD values of less than 2% for routine analysis of lidocaine and nifedipine. This method provides a reliable and effective approach for the simultaneous estimation of lidocaine and nifedipine, contributing to the quality control and safety assessment of pharmaceutical formulations containing these compounds.
Introduction
Anal fissures are tears or fissures in the anal canal's lining of the anal canal that result in discomfort and rectal bleeding during bowel movements. Anal trauma, especially when straining to pass solid faces, can cause them, and they can heal quickly or slowly. Stool softeners, fiber supplements, topical ointments, and, in certain circumstances, surgery are available as treatment.[1] An additional element of the fixed combination, a common local anaesthetic for anal fissures and symptomatic haemorrhoids, is lidocaine.[2] Lidocaine and nifedipine work in complimentary ways when combined. Clinical trials have demonstrated that the anal sphincter's smooth muscle relaxed as a result of nifedipine calcium channel blockage, reducing pain during haemorrhoidectomy [3], [4]. When administered anorectally, this fixed combination was safe, effective, and associated with very few adverse effects, according to clinical tests involving patients with hemorrhoidal thrombosis and anal fissures.[5] Topical nifedipine and diltiazem showed impressive effectiveness, with a healing rate of up to 95% for nifedipine and 67% for diltiazem. Diltiazem and nifedipine are calcium channel blockers with the greatest evidence in favor of topical use. They are made on the spot using bases of cream, gel and ointment. Research has indicated that calcium channel blockers use topically and orally relax the internal anal sphincter, hence reducing the anal resting pressure. While calcium channel blockers and glyceryl trinitrate were tested for their efficacy in treating anal fissures, nifedipine treatment resulted in high healing rates (89%) that were similar to previously reported rates in (95%).[6] Local anaesthetic, which is another part of the fixed combination lidocaine, is typically used to treat symptomatic haemorrhoids and anal fissures.[7] Lidocaine (LID) has the formula 2-(di-ethyl amino)-N-(2,6-di-methylamino) acetamide is a local anaesthetic that has strong anticonvulsant and antiarrhythmic properties. It has sedative, analgesic, and anticonvulsant properties in addition to being a CNS depressant. Nifedipine (NIF) is 3,5-di-methyl-2,6-di-methyl-4-(2-nitrophenyl)-1,4-di-hydropyridine-3,5-di-carboxylate is a strong vasodilator that acts by antagonistically interacting with calcium. It is a helpful blood pressure-lowering antianginal drug. On February 26, 2009 (by CDSCO).[8], [9], [10] The validation has been performed as per ICH Q2(R1) guidelines. A literature survey reveals that lidocaine has been estimated using a number of techniques, including UV spectroscopy, RP-HPLC, HPLC-MS/MS spectrometry, and stability-indicating techniques HPLC, Ultra performance Liquid chromatography (UPLC), MS/MS and UV spectroscopy are techniques for estimating nifedipine, the simultaneous estimation of lidocaine and nifedipine has been done in several investigations using a UV spectroscopic method. Proposed RP-HPLC method includes a separation of drugs at less time.[11], [12], [13], [14], [15], [16], [17], [18], [19]
Objectives
To select the detection wavelength for simultaneous estimation of Lidocaine and Nifedipine
To develop the optimum Mobile phase for the simultaneous estimation of Lidocaine and Nifedipine
To validated the developed method as per ICH Q2 (R1) guidelines for the simultaneous estimation of Lidocaine and Nifedipine.
Material and Methods
Chemicals and reagents
API of Nifedipine was purchased from Mumbai, API of lidocaine was a gift from Valsad, Gujarat. HPLC-grade water and methanol has been purchased from India.
Instrumentation
Thermo Ultimate 3000 (chromeleon software) with a PDA detector C18 column (150 × 4.6 mm × 5 µm) was used. Weighing Balance (XB-220A) Precisa. Digital ultra-sonication cleaner (CD-4820) by Euipton Digital pH meter (S-90) Systonic. UV-Visible spectrophotometer (CARRY-60) Agilent.
Selection of wave length
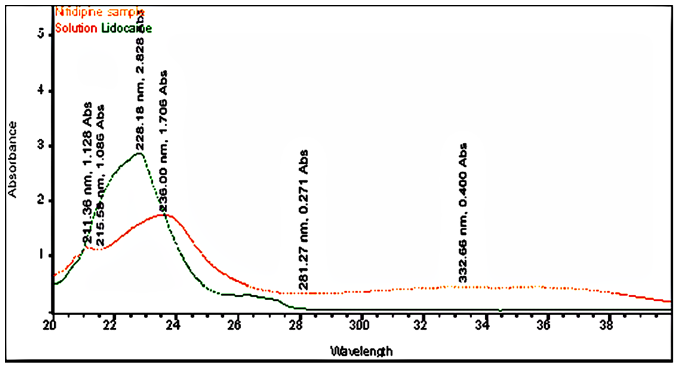
Lidocaine and Nifedipine are soluble in methanol, The standard stock solution 10 µg/mL of lidocaine and nifedipine was prepared in methanol, and the solution scanned in the UV range of 200-400 nm using methanol as a blank, and the UV spectrum was obtained. The absorbance maximum was found to be 236 nm and selected as the detection wavelength for further analysis.
Sample preparation
Lidocaine (LID) and Nifedipine (NIF) standard stock solution were made separately by dissolving the required quantity of each substance in methanol. These solutions were subsequently diluted to create concentration LID at 150 µg/mL and NIF at 30 µg/mL. A quantity equal to 150 mg NIF and 30 mg of LID was precisely weighed and then put to a volumetric flask of 100 ml for the cream sample that included both LID (1.5 %w/w) and NIF (0.3 %w/w). The mixture was then swirled for 30 min and sonicated for 15 min to ensure that the compounds were well dissolved before 5 ml of methanol was infused into the flask. To create a homogeneous solution, 100 ml of methanol were added to the capacity to make it larger. A 0.45 µ nylon syringe filter was used to filter the mixture.
Chromatographic Conditions
Stationary Phase: Thermo Ultimate (chromeleon software) C18 (150 mm × 4.6 mm × 5 µm)
Mobile phase: methanol and water (80:20% v/v)
Flow rate: 1 mL/min
Wavelength: 236 nm
Run time: 10 min.
|
Parameters |
Mean ± SD of Lidocaine |
% RSD |
Mean ± SD of Nifedipine |
% RSD |
|
Area |
217246.2 ± 77.07464 |
0.0354 |
4666849 ± 2305 |
0.0420 |
|
No. of theoretical plates |
5646.333 ± 8.485281 |
0.1502 |
14992 ± 12.727 |
0.0848 |
|
Tailing factor |
1.54 ± 0.007 |
0.4591 |
1.156667 ± 0.0155 |
1.0909 |
|
Parameters |
Lidocaine |
Nifedipine |
|
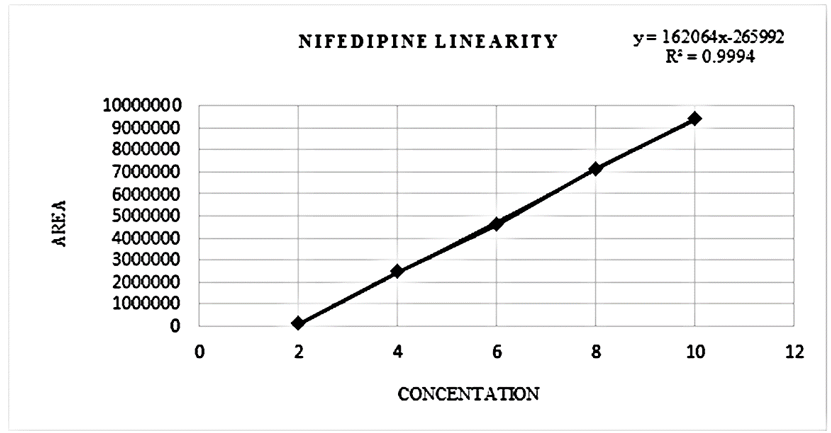
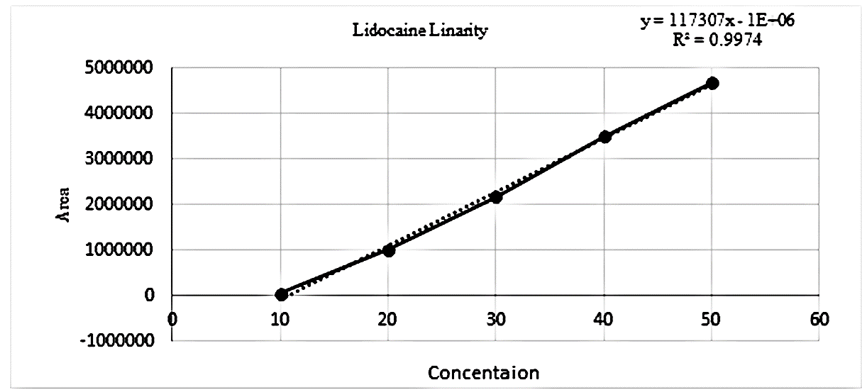
Linearity |
10-50 µg/ml |
2-10 µg/ml |
|
Y – intercept ± SD |
y = 16256x - 206543 |
y =162064x- 265992 |
|
Regression coefficient (R2) |
0.9974 |
0.9994 |
|
LOD |
0.04 µg/ml |
0.03 µg/ml |
|
LOQ |
0.15 µg/ml |
0.10 µg/ml |
|
% Level |
Amount present (µg/mL) |
Amount recovered |
% Recovery |
|||
|
LID |
NIF |
LID |
NIF |
LID |
NIF |
|
|
50 % |
15 |
3 |
15.01 |
3.01 |
100.10 |
100.51 |
|
100 % |
30 |
6 |
30.19 |
6.03 |
100.66 |
100.58 |
|
150 % |
45 |
9 |
45.18 |
8.82 |
100.42 |
98.11 |
|
Drug |
Concentration (µg/ml) |
Intraday % RSD |
Intraday % RSD |
Inter-day % RSD |
Inter-day %RSD |
|
Lidocaine |
15 |
0.4855 |
0.7518 |
0.4855 |
1.056 |
|
30 |
0.1095 |
1.7773 |
0.1095 |
0.350 |
|
|
45 |
0.3545 |
0.067 |
0.3545 |
0.0672 |
|
|
Nifedipine |
3 |
0.0038 |
0.5943 |
0.0038 |
0.0280 |
|
6 |
0.0579 |
0.3974 |
0.0579 |
0.8328 |
|
|
9 |
0.0737 |
0.0524 |
0.0737 |
0.1813 |
|
Parameter |
Flow rate (ml/min) |
Temperature (°c) |
Wavelength (nm) |
|||
|
0.9 |
1.1 |
20 |
30 |
232 |
240 |
|
|
Area of Lidocaine |
232837 |
196493 |
234742 |
246884 |
424803 |
406836 |
|
232283 |
195325 |
234207 |
246321 |
427177 |
406448 |
|
|
233964 |
199236 |
235832 |
248030 |
425136 |
406505 |
|
|
Average |
234284.6 |
196709 |
234992.2 |
247147 |
425098 |
406618 |
|
% RSD |
0.4346 |
0.0830 |
0.1397 |
0.1399 |
0.1379 |
0.0213 |
|
Area of Nifedipine |
5131095 |
4159531 |
4960059 |
5216614 |
9772258 |
8980046 |
|
5119872 |
4140472 |
4949209 |
5205203 |
9760349 |
8965137 |
|
|
5146297 |
4148141 |
4974754 |
5232068 |
9744661 |
8968357 |
|
|
Average |
5134489 |
4154416 |
49633.4 |
5220064 |
9758468 |
8971742 |
|
% RSD |
0.1559 |
0.1559 |
0.1558 |
0.1559 |
0.1132 |
0.0431 |
|
Drugs |
Concentration (µg/ml) |
Area (std) |
% RSD |
Area (sample) |
%W/W |
|
Lidocaine |
30 |
216839 |
1.4823 |
226880 |
|
|
216448 |
216617 |
98.73% |
|||
|
226508 |
216205 |
|
|||
|
Nifedipine |
6 |
4780046 |
0.1709 |
4877047 |
99.32% |
|
4665137 |
4768460 |
||||
|
4768357 |
4760436 |
Method Validation
The validation of proposed methods involved method development, optimization, and adherence to the guidelines specified in ICH Q2 (R1). Following these guidelines ensures the specificity, accuracy, a nd reproducibility of the method, allowing for robustness and credible analytical results.[20], [21] The Linearity has been performed by five injections of standard nifedipine and lidocaine at concentration range of 2-10 µg/mL and 10-50 µg/mL, respectively. Accuracy study has been performed at 50%, 100% and 150%. A study of the precision of the developed method was conducted on both an intra-day and inter-day basis with lidocaine and nifedipine at three distinct concentration levels. The concentrations used were 15 μg/ml, 30 μg/ml, 45 μg/ml, 3 μg/ml, 6 μg/ml, and 9 μg/ml. Robustness has been performed by changing flow rate, temperature and wavelength. Assay has been performed in a sample solution containing 150 µg/mL of lidocaine and 30 µg/mL of nifedipine.
Results and Discussion
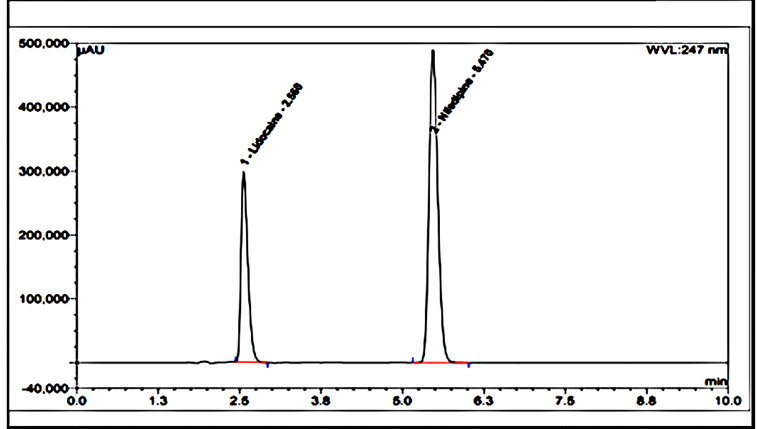
There is an HPLC method reported. I decided to develop a new method by modifying the reported method. Methanol: Water (80:20% v/v) was chosen because it provided peaks with separation of lidocaine and nifedipine. The drugs UV spectra (λ Max) were analysed in the wavelength range of 200-400 nm, and the response for optimization was compared. A careful evaluation of both drugs revealed that 236 nm was the optimal wavelength for adequate sensitivity. The findings obtained the specified requirements, with a resolution value greater than 2, a tailing factor less than 1, and an RSD less than 2. Lidocaine and nifedipine were evaluated using the system suitability test. The total theoretical plates (N) for lidocaine and nifedipine were 5648 and 15000, respectively. These results show effective chromatographic separation and decent peak shape because they are both above the permissible limit of 2000. Lidocaine and nifedipine had tailing factors of 1.356 and 1.094, respectively. Both of these numbers fall below the 2.0 limit, indicating peaks that are symmetrical. The results of the system suitability test for lidocaine and nifedipine are described in [Table 1]. LOD and LOQ for lidocaine and nifedipine were found to be 0.04 and 0.15 for lidocaine and 0.03 and 0.10 for nifedipine, respectively. The results of LOD and LOQ for lidocaine and nifedipine are described in [Table 2] . Standard solutions containing lidocaine (10-50g/ml) and nifedipine (2-10g/ml) were tested to determine linearity. The results of linearity for lidocaine and nifedipine are described in figures 4, 5, and 6. Lidocaine and nifedipine % RSD and % recovery at each concentration level revealed that they were found to be within the range. A range of 99.99% to 100% has been obtained for both lidocaine and nifedipine recovery rates. The results of accuracy are described in[Table 3]. The % RSD has determined at each concentration level being below 2%. Indicates that the development method exhibits high precision, as the RSD value is within the acceptable range. In the robustness study, RSD was found to be less than 2%. In this case, we found the content % to be between 99% and 100%.
Conclusion
The developed RP-HPLC method offers a simple, precise, and accurate tool for simultaneous estimation of lidocaine and nifedipine, application to both bulk drug and topical dosage form analysed, ensuring reliability in pharmaceutical quality control.
Source of Funding
None.
Conflict of Interest
None.
References
- Lund J, Scholefield J. Aetiology and treatment of anal fissure. Br J Surg. 1996;83(10):1335-79. [Google Scholar]
- Carapeti E, Kamm M, Evans B, Phillips R. Topical diltiazem and bethanechol decrease anal sphincter pressure without side effects. Gut. 1999;45(5):719-41. [Google Scholar]
- Cook T, Brading A, Mortensen N. Differences in contractile properties of anorectal smooth muscle and the effects of calcium channel blockade. Br J Surg. 1999;86(1):70-5. [Google Scholar]
- Perrotti P, Bove A, Antropoli C, Molino D, Antropoli M, Balzano A. Topical nifedipine with lidocaine ointment vs. active control for treatment of chronic anal fissure: results of a prospective, randomized, double-blind study. Dis Colon Rectum. 2002;45(11):1468-75. [Google Scholar]
- Kujur A, Ekka P, Chandra N, Lal S, Malua S. Comparative study to assess the effectiveness of topical nif edipine and diltiazem in the treatment of chronic anal fissure. J Family Med Prim Care. 2020;30(11):5652-9. [Google Scholar]
- Perrotti P, Dominici P, Grossi E, Antropoli C, Giannotti G, MC. Pharmacokinetics of anorectal nifedipine and lidocaine (lignocaine) ointment following haemorrhoidectomy: an open-label, single-dose, phase IV clinical study. Clin Drug Invest. 2009;29(4):243-56. [Google Scholar]
- Grigoriev A, Nikitina A, Yaroshenko I, Sidorova A. Development of a HPLC-MS/MS method for the simultaneous determination of nifedipine and lidocaine in human plasma. J Pharm Biomed Anal. 2016;30(131):13-22. [Google Scholar]
- Perrotti P, Bove A, Antropoli C, Molino D, Antropoli M, Balzano A. Topical nifedipine with lidocaine ointment vs. active control for treatment of chronic anal fissure: results of a prospective, randomized, double-blind study. Dis Colon Rectum. 2002;45:1468-75. [Google Scholar]
- Bhusal P, Sharma M, Harrison J, Procter G, Andrews G, Jones D. Svirskis D. Development, validation and application of a stability indicating HPLC method to quantify lidocaine from polyethylene-co-vinyl acetate (EVA) matrices and biological fluids. J Chromatograph Sci. 2017;55(8):832-40. [Google Scholar]
- Furberg C, Bruce M, Psaty J. Nifedipine: dose-related increase in mortality in patients with coronary heart disease. Circ. 1995;92(5):1326-31. [Google Scholar]
- Kumar B, Rajan T. Analytical method development and validation of Lidocaine in ointment formulation by UV spectrophotometric method. Int J Pharm Pharm Sci. 2012;4(2):610-4. [Google Scholar]
- Júnior E, Bentley M, Marchetti J. HPLC assay of lidocaine in in vitro dissolution test of the Poloxamer 407 gels. Rev de Cienc Farm. 2002;38(1):107-18. [Google Scholar]
- Wasan E, Zhao J, Poteet J, Mohammed M, Syeda J, Orlowski T. Development of a UV-stabilized topical formulation of nifedipine for the treatment of Raynaud phenomenon and chilblains. Pharm. 2019;11(11). [Google Scholar]
- Kowalczuk D, Wawrzycka M, Maj A. Application of an HPTLC densitometric method for quantification and identification of nifedipine. J Liq Chromatogr Relat. 2006;29(19):2863-73. [Google Scholar]
- Bhardwaj S, Dwivedia K, Agarwala D. A review: HPLC method development and validation. Int J Snal Bioanal Chem. 2015;5(4):76-81. [Google Scholar]
- Vidushi Y, Meenakshi B, Bharkatiya M. A review on HPLC method development and validation. Pharm Chem Sci. 2017;2(6). [Google Scholar]
- Grigoriev A, Nikitina A, Yaroshenko I, Sidorova A. Development of a HPLC-MS/MS method for the simultaneous determination of nifedipine and lidocaine in human plasma. J Pharm Biomed Anal. 2016;30(131):13-22. [Google Scholar]
- Meshram D, Mehta K, Mishra P. Stability indicating analytical method for the simultaneous estimation of lidocaine and nifedipine in the combined dosage form. Der Pharma Chem. 2018;10(1):60-6. [Google Scholar]
- Yadlapalli S, Katari N, Surya S, Karra V, Kommineni V, Jonnalagadda S. Simultaneous quantification of lidocaine and prilocaine in human plasma by LC-MS/MS and its application in a human pharmacokinetic study. Pract Lab Med. 2019;17. [Google Scholar]
- Guideline I. Validation of analytical procedures: Methodology. Int Confe Harmon. 1996. [Google Scholar]
- . Validation of analytical procedures: text and methodology. Q2 (R1). . 2005. [Google Scholar]
How to Cite This Article
Vancouver
Shinde R, Chandarana C. Development and validation of RP-HPLC method for simultaneous estimation of lidocaine and nifedipine in pure and combined dosage form [Internet]. Int J Pharm Chem Anal. 2024 [cited 2025 Oct 11];11(4):341-345. Available from: https://doi.org/10.18231/j.ijpca.2024.049
APA
Shinde, R., Chandarana, C. (2024). Development and validation of RP-HPLC method for simultaneous estimation of lidocaine and nifedipine in pure and combined dosage form. Int J Pharm Chem Anal, 11(4), 341-345. https://doi.org/10.18231/j.ijpca.2024.049
MLA
Shinde, Rupali, Chandarana, Chandni. "Development and validation of RP-HPLC method for simultaneous estimation of lidocaine and nifedipine in pure and combined dosage form." Int J Pharm Chem Anal, vol. 11, no. 4, 2024, pp. 341-345. https://doi.org/10.18231/j.ijpca.2024.049
Chicago
Shinde, R., Chandarana, C.. "Development and validation of RP-HPLC method for simultaneous estimation of lidocaine and nifedipine in pure and combined dosage form." Int J Pharm Chem Anal 11, no. 4 (2024): 341-345. https://doi.org/10.18231/j.ijpca.2024.049
