- Visibility 365 Views
- Downloads 35 Downloads
- Permissions
- DOI 10.18231/j.ijpca.2022.009
-
CrossMark
- Citation
Design, synthesis and anticancer activity studies of some novel 1,2,4 triazole pyridine derivatives
- Author Details:
-
Namrata Patel
-
Anup K Chakraborty *
-
Sarita Karole
-
Kavita R. Loksh
Abstract
Background: The development of new anticancer agents is one of the most pressing research areas in medicinal chemistry and medicine. The importance of triazole and pyridine rings as scaffolds present in a wide range of therapeutic agents has been well reported and has driven the synthesis of a large number of novel anticancer agents. The presence of these heterocyclic furnishes extensive synthetic possibilities due to the presence of several reaction sites. Prompted by these data we designed, synthesized and evaluated a series of novel 1, 2, 4-triazole-pyridine hybrid derivatives as potential anticancer agents.
Aims and Objectives: To design and synthesize series of novel 1, 2, 4-triazole-pyridine hybrid derivatives as potential anticancer agents
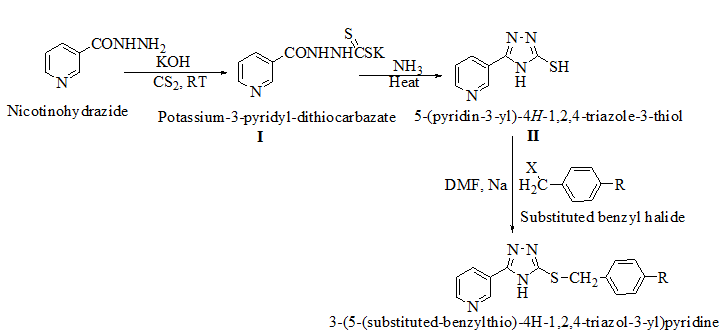
Materials and Methods: Derivatives were synthesized by the reaction of nicotinohydrazide with carbon disulfide to yield potassium-3-pyridyl-dithiocarbazate (I). This was further cyclized with ammonia solution to yield 5-mercapto-substituted 1, 2, 4-triazole-pyridine hybrid (II). This was finally reacted with different substituted benzyl derivatives to produce 1, 2, 4-triazole-pyridine hybrid derivatives (III). The purity of the derivatives was confirmed by thin-layer chromatography and melting point. Structure of these derivatives was set up by determining its infrared spectroscopy, nuclear magnetic resonance spectroscopy and mass spectroscopy. Further, the synthesized 1, 2, 4 triazole pyridine derivatives were tested for their in vitro anticancer activities against murine melanoma (B16F10) using the MTT reduction assay method.
Results: The cell viability study of synthesized compounds concludes that all compounds have moderate to potent anticancer activities against cancer cell lines. Compounds TP1-TP7 have IC50 in the range of 41.12μM to 61.11μM and the highest activity was observed for compound TP6 against murine melanoma (B16F10) cell line.
Conclusion: The present research may pave a way for the development of 1, 2, 4-triazole-pyridine as novel anticancer agents with good efficacy and lesser adverse effects.
Introduction
Cancer is a deadly illness characterized by unregulated cell proliferation and fast spread of aberrant cells.[1] Several environmental and internal variables contribute to aberrant cell line proliferation and the development of various malignancies.[2], [3], [4] Chemotherapy, radiation, and surgery are the three types of cancer treatments now accessible.[5] Chemotherapy is the conventional treatment for cancer patients, in which various chemotherapeutic chemicals are employed to kill cancer cells while causing little harm to normal kidney cells.[6], [7] Nitrogen atoms contain heterocyclic ring moieties, which are found in both natural and synthetic derivatives and have been shown to have powerful anticancer properties against a variety of human cancer cell lines.[8] Three nitrogen atoms with a heterocyclic ring, such as 1,2,4-triazoles, play a vital role in the structural elucidation of numerous natural products[9] and can establish hydrogen bonds with appropriate targets, increasing pharmacokinetics, pharmacological, and toxicological aspects.[10], [11] Anticancer, antibacterial, antitubercular, antifungal, antiviral, analgesic, anti-inflammatory, and tubulin inhibitor properties are all connected with these 1,2,4-triazole compounds. Letrozol [12] is an aromatase inhibitor-containing triazole structural unit used to treat cancer. Researchers are currently concentrating their efforts on developing superior, synergistic compounds by combining two or more active biomolecules or ligands to create novel derivatives with good pharmacological action.[13] Triazole is an appealing bridge group that could be used to link two pharmacophores to create novel bifunctional compounds, despite being nearly hard to hydrolyze, oxidise, or decrease.[14] By combining the pharmacophore including 1,2,4-triazoles and substituted benzyl groups via thio linkage, we were able to generate a small library based on the literature data and the attributes outlined earlier. The antitumor efficacy of a new series of 1, 2, 4-triazole-pyridine hybrid compounds was investigated.
Materials and Methods
All reagents and chemicals were bought for a synthetic purpose from Loba Chemie and Sigma Aldrich. The open capillary method was used to determine the melting point of the produced compounds, and the results were uncorrected. The combustion analysis was used to verify the purity of the synthesised derivatives. IR spectra (KBr, cm-1) (Perkins Elmer Infrared-283 FTIR), 1H NMR (CDCl3) spectra (Brooker 300 MHz spectrometer using tetra methyl silane as an internal reference), and mass spectra were used to validate the structure of the compounds (API 3000 LC-MS).
Synthesis of potassium-3-pyridyl-dithiocarbazate (I)
In 200 mL 100% ethanol, a solution of 8.4gm (0.15M) potassium hydroxide, 13.7gm (0.10M) pyridyl-2-carbohydrazide, and 11.4gm (0.15M) carbon disulfide was produced. After that, the mixture was stirred for 12-16 hours. It was then dried at 65°C after being diluted with 200 cc of dry ether. The salts were produced as stated above and yielded a practically quantitative yield, allowing them to be used without further purification.
Synthesis of pyridine linked 1, 2, 4-triazole-3-thiol (II)
A suspension of I (24gm, 0.096M) in 20 ml (0.864M) ammonia and 40 ml water was refluxed for 3 to 4 hours with stirring. The mixture was then placed into ice cold water (100 ml). The white precipitate was obtained and acidified with strong HCl before being filtered and rinsed in cold water.
Synthesis of 1, 2, 4-triazoles linked with substituted benzyl groups through thio linkage (III)
A solution of sodium (0.14gm, 6M) in dry methanol was mixed with II (0.006M), (0.69gm, 6M) in dry N, N-dimethyl formamide. The benzyl halide (6M) was added after 10 minutes of stirring at room temperature. The resulting suspension was stirred at room temperature for 1-23 hours with a CaCl2 guar tube. TLC was used to confirm the reaction's completion, and the resulting solution was then poured onto crushed ice.
Anticancer activity Screening
MTT assay
Each well of the first plate had the test ingredients removed. Then 50l of MTT reagent (5 mg/ml) was added and incubated in the CO2 incubator for 2 hours at 37°C. After that, the MTT solution was removed and 100 litres of isopropanol were added. To dissolve the formations of purple crystal formazan, the plates were shaken. A microplate reader was used to measure the absorbance at a wavelength of 570nm. Cell viability assay method was used to test the anticancer activities of several produced 1, 2, 4 triazole pyridine derivatives chemicals in vitro.[15] Murine melanoma (B16F10) cancer cell lines were employed to investigate in vitro anticancer activities. The National Center for Cell Science in Pune provided cancer cell lines. The cells were plated or cultured for 24 hours in 96-multiwell plates (104 cells/well). Before being tested for anti-cancerous properties, all of the produced compounds were dissolved in dimethyl sulphoxide. All of the chemicals were applied to the cell monolayer in various concentrations. The vitality of the cells was tested in triplicate for 48 hours using the MTT assay in two distinct dosages of the produced compounds (100M and 10M).
|
Comp. |
R |
Chemical name |
MW/MP |
Appearance / RF |
% yield (w/w)/λ max (nm) |
Solubility |
|
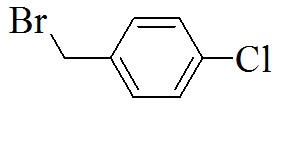
TP1 |
|
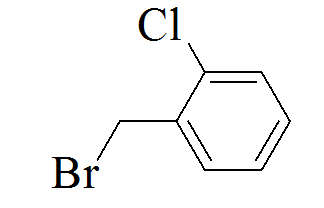
3-(5-(4-chlorobenzylthio)-4H-1,2,4- triazol-3-yl)pyridine |
302/207 |
White solid /0.72 |
73.72/345 |
Ethanol |
|
TP2 |
|
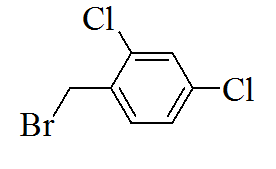
3-(5-(3,4-dichlorobenzylthio)-4H- 1,2,4-triazol-3-yl)pyridine |
337/250 |
Creamy white solid /0.86 |
66.82/350 |
DMF |
|
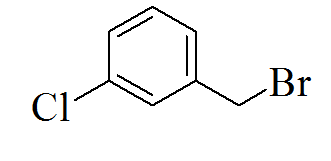
TP3 |
|
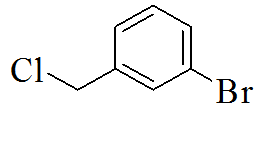
3-(5-(3-chlorobenzylthio)-4H-1,2,4- triazol-3-yl)pyridine |
302/207 |
Light brown solid /0.73 |
70/345 |
Ethanol |
|
TP4 |
|
3-(5-(2-chlorobenzylthio)-4H-1,2,4- triazol-3-yl)pyridine |
302/207 |
Brown solid/0.72 |
73/346 |
Ethanol |
|
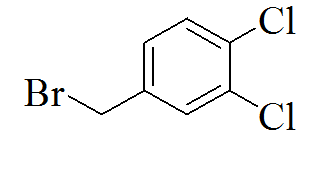
TP5 |
|
3-(5-(2,4-dichlorobenzylthio)-4H- 1,2,4-triazol-3-yl)pyridine |
337/250 |
Yellow solid /0.85 |
66.19/352 |
Ethanol |
|
TP6 |
|
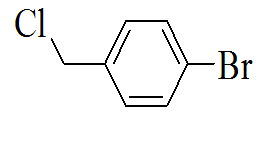
3-(5-(4-bromobenzylthio)-4H-1,2,4- triazol-3-yl)pyridine |
347/537 |
White solid /0.89 |
62.19/342 |
Water |
|
TP7 |
|
3-(5-(3-bromobenzylthio)-4H-1,2,4- triazol-3-yl)pyridine |
347/237 |
Brown solid /0.90 |
70.39/341 |
Water |
|
Combustion analysis |
||
|
Comp. |
Theoretical value |
Observed values |
|
TP1 |
C (55.53%) H (3.66%) Cl (11.7%) N (18.50) S (10.59%) |
C (54.53%) H (3.60%) Cl (11.5%) N (18.21) S (10.49%) |
|
TP2 |
C (55.53%) H (3.66%) Cl (11.7%) N (18.50) S (10.59%) |
C (49.26%) H (2.91%) Cl (20.82%) N (16.27%) S (9.41%) |
|
TP3 |
C (55.53%) H (3.66%) Cl (11.7%) N (18.50) S (10.59%) |
C (54.55%) H (3.60%) Cl (11.38%) N (18.10) S (10.41%) |
|
TP4 |
C (55.53%) H (3.66%) Cl (11.7%) N (18.50) S (10.59%) |
C (55.01%) H (3.59%) Cl (11.38%) N (18.36) S (10.39%) |
|
TP5 |
C (49.86%) H (2.99%) Cl (21.03%) N (16.61%) S (9.51%) |
C (49.06%) H (2.94%) Cl (21.0%) N (16.41%) S (9.41%) |
|
TP6 |
C (48.43%) H (3.19%) Cl (23.01%) N (16.14%) S (9.23%) |
C (48.02%) H (3.16%) Cl (22.91%) N (16.04%) S (9.20%) |
|
TP7 |
C (48.43%) H (3.19%) Cl (23.01%) N (16.14%) S (9.23%) |
C (48.02%) H (3.16%) Cl (22.91%) N (16.04%) S (9.20%) |
|
Comp. |
IR (KBr cm-1) |
1H NMR δ (ppm) (DMSO-d6) |
MASS |
|
TP1 |
2978.85(Ar-C-H str), 1630.41(Ar-C=C str), 1154.87 (Ar–C–C str), 1595.76(C=Nstr), 1252.41(-C-N- str), 657.11(-C-S str), 735.83(C-Cl str) |
6.84-7.19 (m 4H, Ar-H), 4.25 (s 2H, -CH2), 7.40-8.90 (m 4H, pyridine ring) |
301+ |
|
TP2 |
3088.25(Ar-C-H str), 1610.41(Ar-C=C str), 1173.78 (Ar–C–C str), 1542.56(C=Nstr), 1200.41(-C-N- str), 647.12(-C-S str), 717.33(C-Cl str) |
6.88-7.29 (m 4H, Ar-H), 4.19 (s 2H, -CH2), 7.44-8.86 (m 4H, pyridine ring) |
336+ |
|
TP3 |
3108.64(Ar-C-H str), 1684.40(Ar-C=C str), 1112.08 (Ar–C–C str), 1521.24(C=Nstr), 1221.56(-C-N- str), 612.41.11(-CS str), 712.98(C-Cl str) |
6.94-7.46 (m 4H, Ar-H), 4.21 (s 2H, -CH2), 7.74-8.78 (m 4H, pyridine ring) |
301+ |
|
TP4 |
2968.46(Ar-C-H str), 1598.35.47(Ar-C=C str), 1175.47 (Ar–C–C str), 1500.36(C=Nstr), 1285.47(-C-N- str), 611.81(-C-S str), 765.79(C-Cl str) |
7.02-7.27 (m 4H, Ar-H), 4.20 (s 2H, -CH2), 7.63-8.63 (m 4H, pyridine ring) |
301+ |
|
TP5 |
2912.56(Ar-C-H str), 1623.56(Ar-C=C str), 1121.67(Ar–C–C str), 1521.12(C=Nstr), 1213.87(-C-N- str), 641.78(-C-S str), 735.13(C-Cl str) |
6.64-7.10 (m 3H, Ar-H), 4.12 (s 1H, -CH2), 7.30-8.86 (m 4H, pyridine ring) |
336+ |
|
TP6 |
2890.45(Ar-C-H str), 1611.76(Ar-C=C str), 1108.45(Ar–C–C str), 1541.10(C=Nstr), 1286.45(-C-N- str), 698.34(-C-S str), 812.12(C-Br str) |
6.94-7.30 (m 4H, Ar-H), 4.15 (s 2H, -CH2), 7.60-8.86 (m 4H, pyridine ring) |
346+ |
|
TP7 |
2890.45(Ar-C-H str), 1611.76(Ar-C=C str), 1108.45(Ar–C–C str), 1541.10(C=Nstr), 1286.45(-C-N- str), 698.34(-C-S str), 812.12(C-Br str) |
7.10-7.30 (m 4H, Ar-H), 4.19 (s 2H, -CH2), 7.44-8.84 (m 4H, pyridine ring) |
346+ |
|
S. No |
Compounds |
B16F10 (μM) |
|
1 |
TP1 |
58.50 |
|
2 |
TP2 |
52.35 |
|
3 |
TP3 |
57.70 |
|
4 |
TP4 |
50.25 |
|
5 |
TP5 |
61.11 |
|
6 |
TP6 |
41.12 |
|
7 |
TP7 |
45.60 |
The MTT (3-(4, 5- dimethylthiazol-2-yl) 2, 5 diphenyltetrazolium bromide) assay was used to determine the vitality of the cells. This assay employed MTT reagent [3-(4, 5-dimethylthiazol-2-yl)-2, 5- diphenyltetrazolium bromide] at a concentration of 5 mg/ml. The anticancer efficacy of these drugs was determined using the IC50 method (the concentration that causes a 50 percent reduction of the cell growth).
Results and Discussion
We created a pool of compounds with a combined pharmacophore of 1, 2, 4-triazoles and substituted benzyl groups in the quest for new anticancer drugs. TLC, combustion analysis, and different spectroscopic techniques were used to examine the newly synthesised fused pharmacophore. The MTT test method was used to assess the anticancer activity of the new compounds.[Figure 1] shows the methodologies for synthesising unique merging pharmacophores of 1, 2, 4-triazoles and substituted benzyl groups. By treating 5-mercapto-3-pyridyl-1, 2, 4-triazole with variously substituted benzyl halides, a pool of seven distinct merging pharmacophores was created.[Table 1] Displayed the chemical structure, melting point, and other physical data, whereas Table 2 displayed the results of the combustion analysis. The IR, 1H NMR, and MS spectrum of several 1,2,4-triazoles coupled with substituted benzyl groups via thio linkage derivatives were recorded to demonstrate their formation. The infrared spectra of 3-(5-(4-chlorobenzylthio)-4H-1,2,4-triazol-3-yl) pyridine, a 1,2,4-triazole derivative, displayed a strong C=N stretching (str) band at 1595.76 cm-1 and a C-N absorption band at 1252.41 cm-1, indicating ring closure of the 1,2,4-triazole ring. Aromatic (Ar) C-H str has an absorption band at 2978.85 cm-1, C=C str has an absorption band at 1630.41 cm-1, C-Cl str has an absorption band at 735.83 cm-1, and C-S str has an absorption band at 657.11 cm-1. All derivatives had strong absorption at roughly 3078.85 and 1620.47 cm-1, which was confirmed for aromatic C-H and C=C bonds, respectively. 1H NMR results revealed the existence of certain functional groups in produced derivatives. The four aromatic proton, 7.40-8.90 is due to four pyridine proton, and 4.25 is due to two methylene proton in the 1H NMR spectrum of triazole derivatives 3-(5-(4-chlorobenzylthio)-4H-1,2,4-triazol-3-yl)pyridine. Shift value 3.67 confirmed the presence of another group, such as -CH3 manufactured derivatives. The mass spectra of the triazole derivative 3-(5-(4-chlorobenzylthio)-4H-1,2,4-triazol-3-yl)pyridine revealed a molecular ion peak at m/z 301+, which matches the molecular formula C14H11ClN4S. Table 3 also contains spectral data for the remaining derivatives. The MTT assay was used to investigate the in vitro anticancer properties of the synthesized 1, 2, 4 triazole pyridine derivatives against murine melanoma (B16F10). Table 4 summarizes the assay results given as IC50 (M). The IC50 value is the average of three separate experiments and indicates the concentration of a substance that inhibits cell growth by 50% after 48 hours of incubation. Because the results indicate that all of the tested compounds have potential, they were chosen for the measurement of IC50 values, or the concentration required inhibiting cancer cells by 50% when treated with manufactured compounds. According to the results of the cell viability research, all produced compounds have moderate to powerful anticancer activity against cancer cell lines. Compounds TP1-TP7 exhibit IC50s ranging from 41.12M to 61.11M, with compound TP6 having the highest activity against the murine melanoma (B16F10) cell line.
Conclusion
By treating pyridine linked 1,2,4-triazole-3-thiol with different substituted benzyl halides, a new series of diverse 1,2,4-triazoles connected with substituted benzyl groups through thio linkage derivatives was created, using a simple, appropriate, and well-organized synthetic approach. TLC, IR, NMR, and MS were used to confirm the physical and analytical properties of the newly synthesized 1, 2, 4-triazole derivatives. Following that, pharmacological testing revealed that the derivative 3-(5-(4-bromobenzylthio)-4H-1, 2, 4- triazol-3-yl) pyridine had more anticancer activity than other compounds. As a result, we believe that the findings of this study could open the way for the creation of innovative anticancer drugs with high efficacy and fewer side effects.
Source of Funding
None.
Conflict of Interest
None.
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69-90. [Google Scholar] [Crossref]
- Park SK, Cho LY, Yang JJ, Park B, Chang SH, Lee KS. Lung cancer risk and cigarette smoking, lung tuberculosis according to histologic type and gender in a population based case-control study. Lung Cancer. 2010;68(1):20-6. [Google Scholar] [Crossref]
- Clayton PE, Banerjee I, Murray PG, Renehan AG. Growth hormone, the insulin-like growth factor axis, insulin and cancer risk. Nat Rev Endocrinol. 2011;7(1):11-24. [Google Scholar] [Crossref]
- Porta C, Riboldi E, Sica A. Mechanisms linking pathogensassociated inflammation and cancer. Cancer Lett. 2011;305(2):250-62. [Google Scholar] [Crossref]
- Khan FA, Akhtar SS, Sheikh MK. Cancer treatment-objectives and quality of life issues. Malays J Med Sci. 2005;12(1):3-5. [Google Scholar]
- Rebucci M, Michiels C. Molecular aspects of cancer cell resistance to chemotherapy. Biochem Pharmacol. 2013;85(9):1219-26. [Google Scholar] [Crossref]
- Rosa R, Monteleone F, Zambrano N, Bianco R. In vitro and in vivo models for analysis of resistance to anticancer molecular molecular therapies. Curr Med Chem. 2014;21(14):1595-1606. [Google Scholar]
- Pragathi RYJ, Sreenivasulu D. Rudraraju Ramesh Raju. Design, Synthesis, and Biological Evaluation of 1, 2, 4-Thiadiazole-1, 2, 4-Triazole Derivatives Bearing Amide Functionality as Anticancer Agents. Arabian J Sci Eng. 2021;46:225-32. [Google Scholar]
- Asami T, Min YK, Nagata N, Amagishi KY, Takatsuto S, Fujioka S. Characterization of brassinazole, a triazole-type brassinosteroid biosynthesis inhibitor. Plant Physiol. 2000;123(1):93-100. [Google Scholar] [Crossref]
- Kaur P, Chawla A. Hepatoprotective activity of Inula cappa DC. Aqueous extract against carbon tetrachloride induced hepatotoxicity in Wistar rats. Int Res J Pharm. 2017;8(1):14-9. [Google Scholar] [Crossref]
- Kapron B, Luszczki JJ, Plaziska A, Siwek A, Karcz T, Grybos A. Development of the 1,2,4-triazole-based anticonvulsant drug candidates acting on the voltage-gated sodium channels. Insights from in vivo, in vitro, and in silico studies. Eur J Pharm Sci. 2019;129:42-57. [Google Scholar] [Crossref]
- Stresser DM, Turner SD, Mcnamara J, Stocker P, Miller VP, Crespi CL. Ahigh-throughput screen to identify inhibitors of aromatase (CYP19). Anal. Biochem. 2000;284(2):427-30. [Google Scholar] [Crossref]
- Dewangan D, Verma VS, Nakhate K, Tripathi DK, Kashyap P, Dongade H. Synthesis, characterization, and screening for analgesic and anti-inflammatory activities of new 1,3,4-oxadiazole derivatives linked to quinazolin-4-one ring. Med Chem Res. 2016;25(10):2143-54. [Google Scholar] [Crossref]
- Petrova KT, Potewar TM, Silva PC, Barros TM, Calhelha RC, Ciric A. Antimicrobial and cytotoxic activities of 1,2,3-triazole-sucrose derivatives. Carbohydr Res. 2015;417:66-71. [Google Scholar] [Crossref]
- Prakash B, Amuthavalli A, Edison D. Novel indole derivatives as potential anticancer agents: Design, synthesis and biological screening. Med Chem Res. 2018;27:321-31. [Google Scholar]
- Abstract
- Introduction
- Materials and Methods
- Synthesis of potassium-3-pyridyl-dithiocarbazate (I)
- Synthesis of pyridine linked 1, 2, 4-triazole-3-thiol (II)
- Synthesis of 1, 2, 4-triazoles linked with substituted benzyl groups through thio linkage (III)
- Anticancer activity Screening
- Results and Discussion
- Conclusion
- Source of Funding
- Conflict of Interest
- References
How to Cite This Article
Vancouver
Patel N, Chakraborty AK, Karole S, Loksh KR. Design, synthesis and anticancer activity studies of some novel 1,2,4 triazole pyridine derivatives [Internet]. Int J Pharm Chem Anal. 2022 [cited 2025 Oct 04];9(1):56-61. Available from: https://doi.org/10.18231/j.ijpca.2022.009
APA
Patel, N., Chakraborty, A. K., Karole, S., Loksh, K. R. (2022). Design, synthesis and anticancer activity studies of some novel 1,2,4 triazole pyridine derivatives. Int J Pharm Chem Anal, 9(1), 56-61. https://doi.org/10.18231/j.ijpca.2022.009
MLA
Patel, Namrata, Chakraborty, Anup K, Karole, Sarita, Loksh, Kavita R.. "Design, synthesis and anticancer activity studies of some novel 1,2,4 triazole pyridine derivatives." Int J Pharm Chem Anal, vol. 9, no. 1, 2022, pp. 56-61. https://doi.org/10.18231/j.ijpca.2022.009
Chicago
Patel, N., Chakraborty, A. K., Karole, S., Loksh, K. R.. "Design, synthesis and anticancer activity studies of some novel 1,2,4 triazole pyridine derivatives." Int J Pharm Chem Anal 9, no. 1 (2022): 56-61. https://doi.org/10.18231/j.ijpca.2022.009
