- Visibility 205 Views
- Downloads 53 Downloads
- Permissions
- DOI 10.18231/j.ijpca.2021.025
-
CrossMark
- Citation
Method development and validation for simultaneous estimation of amlodipine besylate and enalapril maleate in solid dosage form
- Author Details:
-
Manisha Masih *
Abstract
A rapid, sensitive, specific, accurate and precise high pressure liquid chromatographic method (HPLC) method involving UV detection has been developed for the determination and quantification of Amlodipine Besylate and Enalapril maleate in bulk and combined dosage form. The determination was carried out on a Phenomenex C18 column (Dimention : 250 x 4.6 mm, 5 μm). The sample was analysed using filtered and degassed mixture of methanol : 0.1N HCl (1:1) as mobile phase at a flow rate of 1ml/min and effluent was monitored at 218nm. The retention time for Amlodipine besylate was 7.6 min and for Enalapril maleate 3.2 min. Amlodipine besylate and Enalapril maleate showed a linear response in the concentration range of 10-50μg/ml. The correlation co-efficient ('r' value) for Amlodipine besylate and Enalapril maleate was 0.9992 and 0.9994, respectively. The method was validated in terms of linearity, precision, accuracy, specificity, robustness and solution stability. The proposed method can be used for routine analysis of Amlodipine Besylate and Enalapril maleate in bulk and combined dosage form
Introduction
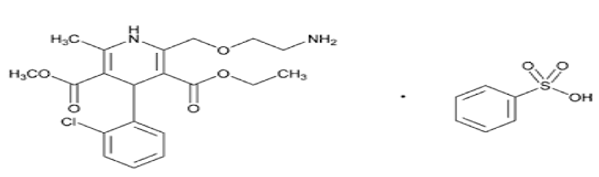
Amlodipine is a synthetic dihydropyridine and a calcium channel blocker with antihypertensive and antianginal properties. It is a dihydropyridine, a member of monochlorobenzenes, an ethyl ester, a methyl ester and a primary amino compound. Chemical name of amlidipine is 3-O-ethyl 5-O-methyl 2-(2-aminoethoxymethyl)-4-(2-chlorophenyl)-6-methyl-1,4-dihydropyridine-3,5-dicarboxylate benzene sulphonate ([Figure 1]). [1], [2] Amlodipine act by blocking voltage-sensitive calcium channels (L-type). Amlodipine slow conduction in the SA and AV nodes where action potential propagation depends on slow inward Ca2+ current, slowing the heart and terminating SVT by causing partial AV block. It shortens the plateau of the action potential and reduces the force of contraction. Reduced Ca2+ entry reduces after depolarization and thus suppresses premature ectopic beats.[3], [4], [5]
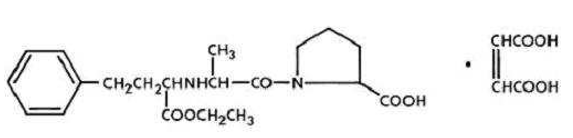
Enalapril is a prodrug which is hydrolysed in the body to Enalaprilate, which is an inhibitor of angiotensin-converting enzyme (ACE). It is indicated for treatment of hypertension, treatment of symptomatic heart failure and prevention of symptomatic heart failure in patients with asymptomatic left ventricular dysfunction (ejection fraction <35%). Chemically it is ((S)-1-{N-[1-(ethoxycarbonyl)-3-phenylpropyl]-Lalanyl}-L-proline, (Z)-2-butenedioate (1:1) ([Figure 2]), a derivative of two amino-acids, L-alanine and L-proline. It is a white to off-white crystalline, odourless powder which melts in the range of 143–144◦C. ACE is a peptidyl dipeptidase that catalyzes the conversion of angiotensin-I to the vasoconstrictor substance, angiotensin-II, which stimulates aldosterone secretion by the adrenal cortex. Blocking the conversion of the angiotensin I to the angiotensin II, leads to a reduction in vasopressin activity and a decrease in peripheral vascular resistance.[6], [7], [8], [9], [10], [11], [12]
Materials and Methods
Reagents and chemicals
All solvents used were of HPLC grade. The reference standards of Amlodipine besylate and Enalapril maleate were obtained as gift samples from LUPIN Pharmaceutical Ltd. (Bhopal, India). The commercial fixed dose combination product Amtas E (Intas, Ahemdabad) containing Amlodipine 5 mg and Enalapril 5 mg was obtained from local pharmacy store. The solvents used were Methanol HPLC grade and Hydrochloric acid was procured from Cipla.
Preparation of standard stock solution
The standard stock solutions of AML (100μg/ml), ENA (100μg/ml) were prepared by transferring 10mg of Amlodipine besylate and 10mg of Enalapril maleate respectively in 100ml Volumetric flasks. The volume was made upto the mark using mobile phase (methanol : 0.1N HCl [1:1]). The solutions were sonicated for 15 min and filtered through Whatmann filter paper.
Preparation of sample solution
Twenty tablets were weighed accurately, their average weight was determined and powdered. The powder of the tablets equivalent to 5 mg of AML and 5 mg of ENA was transferred into 50 ml volumetric flask. 25 ml of methanol : 0.1N HCl (1:1) was added into the volumetric flask and sonicated for 15 min to effect complete dissolution of the drugs. Then the volume was made upto the mark with mobile phase. The solution was filtered through the Whatmann filter paper and the aliquot portion of the filtrate was further diluted to get the final concentration of 100µg/ml. 10μl of the above solution was injected into the HPLC under the set chromatographic conditions.
Instrument and chromatographic conditions
Chromatographic separation was carried out using Analytical Technologies Ltd HPLC system with UV-2230 UV-Vis detector and P-2230 HPLC pump. The elution was carried out isocratically
|
Parameter/Condition |
Specification |
|
Column |
Phenomenex C18 (250 x 4.6 mm, 5 μm) |
|
Mobile phase |
Methanol: 0.1N HCl (1:1) |
|
Flow rate |
1ml/min |
|
Wavelength of detection |
218nm |
|
Sample load |
10 μl |
|
Column temperature |
40ºC |
Results and Discussion


Method validation
Validation of any analytical method shall be done to establish by laboratory studies, that the performance of the method meet the requirement for the intended analytical application. The method was validated according to ICH guidelines to study linearity, accuracy and precision.[13], [14], [15]
Linearity
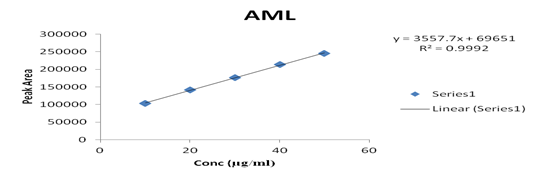
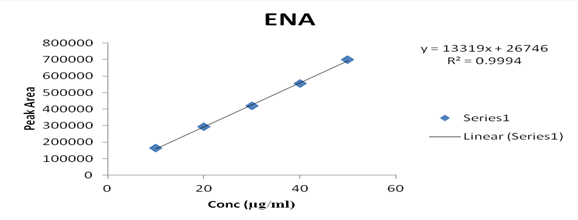
Several aliquots of standard solutions of AML and ENA were taken in different 10 ml volumetric flasks and the volume was made upto the mark with mobile phase such that final concentration of AML and ENA were 10-50 μg/ml, respectively. Evaluation was performed using the UV-Vis detector at 218 nm, peak area recorded for all the peaks, results are displayed in [Table 2]. Calibration curve was plotted as concentration against peak area as shown in graph 2 & 3. The slope and intercept value for calibration curve were y = 3557.7x + 69651 (R² = 0.9992) for AML, y = 13319x + 26746 (R² = 0.9994) for ENA
|
S.No. |
Concentration of Amlodipine besylate (μg/ml) |
Peak Area |
Concentration of Enalapril maleate (μg/ml) |
Peak Area |
|
1 |
10 |
103821 |
10 |
163820 |
|
2 |
20 |
142038 |
20 |
293901 |
|
3 |
30 |
176420 |
30 |
419092 |
|
4 |
40 |
213805 |
40 |
556098 |
|
5 |
50 |
245821 |
50 |
698672 |
Recovery
Accuracy of the method was calculated by recovery studies at three levels (80%, 100% and 120%) by standard addition method. The accuracy was expressed as the percentage of the analyte recovered. Accuracy of proposed method was checked as per ICH guidelines. For AML, tablet powder equivalent to 5 mg AML was taken individually into three different 100 ml volumetric flasks and then 8 mg (80%), 10 mg (100%) and 12 mg (120%) of standard AML were added to each of the volumetric flasks. After that 25 ml of the mobile phase [methanol : 0.1N HCl (1:1)] was added to each of the volumetric flask and sonicated for 5 min. The solutions were then filtered and 1 ml of the filtrate from each was taken in 10 ml volumetric flasks individually and diluted upto the mark with mobile phase. The solutions were injected in triplicates into the chromatographic system and the peak area were evaluated to give Percent Recovery and Standard deviation. Similar procedure was repeated for other drug.
|
Drug |
Intraday |
Interday |
||
|
% Obtained ± SD |
%RSD |
% Obtained ± SD |
%RSD |
|
|
Amlodipine Besylate |
103.72 ± 0.87 |
0.85 |
107.51 ± 1.01 |
0.94 |
|
Enalapril Maleate |
103.54 ± 0.88 |
0.86 |
105.35 ± 1.008 |
0.96 |
Robustness: The robustness of the proposed method was verified by varying the solvent ratio in the mobile phase, flow rate and wavelength range. Sample solutions were injected as 10μl injection into the chromatographic system. The parameters studied were peak area and found their standard deviation & % RSD.
Limit of detection and Limit of quantification: The LOD and LOQ of the proposed method were determined by progressively injecting lower concentrations of the standard solutions under the set chromatographic conditions. The results obtained are displayed in [Table 5].
L.O.D. = 3.3(SD/S)
L.O.Q. = 10(SD/S)
Where, SD = Standard deviation of the response,
S =Slope of the calibration curve. The slope S may be estimated from the calibration curve of the analyte.
|
Drug |
LOD |
LOQ |
|
Amlodipine besylate |
0.14 |
0.42 |
|
Enalapril maleate |
0.05 |
0.15 |
|
Parameters |
Observation |
|
|
Amlodipine besylate |
Enalapril maleate |
|
|
Linearity |
10 – 50µg/ml |
10 – 50µg/ml |
|
Regression equation |
y = 3557.7x + 69651 |
y = 13319x + 26746 |
|
Correlation coefficient |
0.9992 |
0.9994 |
|
Retention time |
7.6 min |
3.2 min |
|
Resolution |
28.11 |
36.14 |
|
Theoretical plates |
29218.64 |
38196.47 |
|
Robustness |
Robust |
Robust |
|
LOD |
0.14 |
0.05 |
|
LOQ |
0.42 |
0.15 |
Conclusion
The developed method gives good resolution between Amlodipine besylate and Enalapril maleate with short analysis time. The method is simple, accurate, rapid, precise and can be easily used for routine analysis of these drugs without involving any complicated sample preparation.
Acknowledgment
The authors are thankful to Cipla Pharmaceuticals for providing the opportunity to carry out the Research work and Lupin Pharmaceuticals Ltd (Bhopal) for providing the gift samples of the Amlodipine besylate and Enalapril maleate respectively.
Source of Funding
None.
Conflict of Interest
None.
References
- Masih M, Mittal A, Nandy BC. Development and Validation of Hplc Method for Simultaneous Estimation of Amlodipine Besylate and Lisinopril Dihydrate in Solid Dosage Form. ASIO J Anal Chem. 2014;1(1):33-7. [Google Scholar]
- Logoyda L. Efficient validated method of HPLC to determine amlodipine in combinated dosage form containing amlodipine, enalapril and bisoprolol and in vitro dissolution studies with in vitro/in vivo correlation. Pharmacia. 2020;67(2):55-61. [Google Scholar]
- Wilson G. Organic Medicinal and Pharmaceutical chemistry ed. . 2004. [Google Scholar]
- Tripathi KD. . Essentials of Medical Pharmacology. 2004. [Google Scholar]
- Garg G, Saraf S, SS. Development and validation of simultaneous estimation of Enalapril maleate and Amlodione Besylate in combined dosage forms. Trends App Sci Res. 2008;3(3):278-84. [Google Scholar] [Crossref]
- Franz DN, Gennaro AR, Remington. The Science and Practice of Pharmacy. . . [Google Scholar]
- Warner GT, Perry CM. Ramipril: a review of its use in the prevention of cardiovascular outcomes. Drugs. 2002;62(9):1381-1405. [Google Scholar] [Crossref]
- Al-Momani. Determination of Hydrochlorothiazide and Enalapril maleate in tablet formulation by RP-HPLC. Turk J Chem. 2001;25(1):49-54. [Google Scholar]
- Strauss R. Enalaprilat in hypertensive emergencies. J Cli Pharmaco. 1986;26(1):39-43. [Google Scholar] [Crossref]
- Tajerzadeh H, Hamidi M. A simple HPLC method for quantitation of enalaprilat. J Pharma Biomed Anal. 2001;24(4):675-80. [Google Scholar] [Crossref]
- Koppala S. User-Friendly HPLC Method Development and Validation for Determination of Enalapril Maleate and Its Impurities in Enalapril Tablets. J Chromato Sci. 2017;55(10):979-88. [Google Scholar]
- . United States Pharmacopeia; Enalapril maleate monograph. . 2016;28. [Google Scholar]
- Sethi PD, Sethi R. HPLC-Quantitative analysis of pharmaceutical formulations. . 1997. [Google Scholar]
- . ICH Harmonised Tripartite Guidelines, Validation of analytical procedures: text & methodology, Q2 (R). . 2005. [Google Scholar]
- . ICH Q1A (R2) Stability testing of new drug substances and drug products . . 2003. [Google Scholar]
How to Cite This Article
Vancouver
Masih M. Method development and validation for simultaneous estimation of amlodipine besylate and enalapril maleate in solid dosage form [Internet]. Int J Pharm Chem Anal. 2021 [cited 2025 Oct 13];8(3):129-133. Available from: https://doi.org/10.18231/j.ijpca.2021.025
APA
Masih, M. (2021). Method development and validation for simultaneous estimation of amlodipine besylate and enalapril maleate in solid dosage form. Int J Pharm Chem Anal, 8(3), 129-133. https://doi.org/10.18231/j.ijpca.2021.025
MLA
Masih, Manisha. "Method development and validation for simultaneous estimation of amlodipine besylate and enalapril maleate in solid dosage form." Int J Pharm Chem Anal, vol. 8, no. 3, 2021, pp. 129-133. https://doi.org/10.18231/j.ijpca.2021.025
Chicago
Masih, M.. "Method development and validation for simultaneous estimation of amlodipine besylate and enalapril maleate in solid dosage form." Int J Pharm Chem Anal 8, no. 3 (2021): 129-133. https://doi.org/10.18231/j.ijpca.2021.025
