- Visibility 137 Views
- Downloads 100 Downloads
- Permissions
- DOI 10.18231/j.ijpca.2022.023
-
CrossMark
- Citation
Chromatographic estimation of cefetamet pivoxil in bulk and tablet dosage form: Development and validation approach
- Author Details:
-
Mahesh Bhanusali
-
M. S. Palled *
-
Shailendra S. Suryawanshi
Abstract
Cefetamet pivoxil is an oral third generation cephalosporin class of antibiotic which is mainly used in the treatment and management of both upper and lower community acquired respiratory tract infection. Many branded and generic formulations are available in tablet dosage forms in market, hence the quality control of Cefetamet pivoxil play an important role in pharmaceutical industry. In the present research work an attempt has been made to develop and validate cost effective, simple, precise, robust and rugged chromatographic method for estimation of Cefetamet pivoxil in bulk and tablets. Chromatographic method was developed using HPLC instrument. The separation was based on isocratic reverse phase chromatographic mode using C-18 column as stationary phase and mobile phase composed of acetonitrile: water (80:20 v/v) at ambient temperature using UV detection at 251 nm. Developed method was validated by analyzing parameters such as specificity, selectivity, linearity, range, precision, robustness, ruggedness, detection limit, quantification limit and drug recovery. All the method validation reportwas found to be within the acceptance as per ICH guidelines. Linearity was found between the range of 10 – 50 mg/ml. The results obtained from the validation data showed that method was specific, selective, linear, precise, accurate, robust and rugged and hence can be used for estimation of Cefetamet pivoxil in bulk and tablets dosage forms.
Introduction
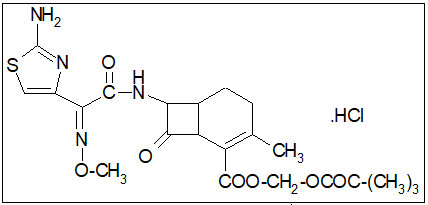
Cefetamet pivoxil is an oral third generation cephalosporin class of antibiotic which is mainly used in the treatment and management of both upper and lower community acquired respiratory tract infection.[1] It has an excellent antimicrobial activity against the major respiratory pathogens such as Streptococcus pneumonia, Haemophilusinfluenzae, Moraxella catarrhalis, Neisseriegonorrhoea and Enterobacteriaceae.[2] Cefetamet pivoxil is used in infection caused by susceptible micro organism found in otitis media, sinusitis, pharyngo tonsillitis, acute exacerbation of chronic bronchitis, tracheobronchitis of bacterial origin pneumonia, complicated and uncomplicated UTI, acute gonococcalurethretis. Many branded and generic formulations are available in market Altamet, Recocef, Bacirom O.
Literature survey has been done on the analytical methods for analysis of Cefetamet pivoxil. The reviewed methods include analysis of Cefetamet pivoxil hydrochloride in drug substance and powder for oral suspension, spectrophotometric estimation of Cefetamet pivoxil in pharmaceutical formulation and reported few chromatographic methods in bulk, powders and other pharmaceutical dosage forms.[3], [4], [5] In the present analytical research, an attempt has been made to develop and validate suitable, simple, precise, robust, rugged and accurate chromatographic RP-HPLC method for estimation of Cefetamet pivoxil in bulk and tables.[6], [7], [8], [9]
Materials and Methods
Drug samples
Cefetamet pivoxil and tablets formulation were procured from Alembic Limited, Vadodara.sa
Chemical and reagents
All the reagents and solvents used are pure and analytical grade and obtained from store house of KLE College of Pharmacy, Belagavi. Analytical grade acetonitrile was obtained from Merck Pvt Ltd Mumbai, HPLC gradewater was obtained from RFCL Ltd New Delhi).
Instruments and apparatus
Lachrom HPLC system consisting of Merck HITACHI pump L-7100, Column oven L-7350 connected with UV detector L-7400 was used for analysis.
Methodology
The separation of analyte in HPLC system was carried on the basis of reverse phase isocratic chromatographic mode. Solubility of Cefetamet was checked in the various polar and non-polar solvent and also through the literature search and solvents was selected for the preparation of mobile phase.
Stationary phase
Hypersil C-18 column was used as stationary phase.
Preparation of mobile phase
Solvent system composed of acetonitrile and water in the ratio 80:20 v/v was prepared, filtered through membrane nylon filters of size 4.5 μ and sonicated for 15 minutes and used for the chromatographic separation.
Selection of wavelength of detection
Solution containing analyte in the mobile phase was scanned in the UV-Spectrophotometer between 400-200 nm and UV spectrum was obtained. Analyte showed maximum absorbance at 251 nm and hence it was used as detection wavelength in HPLC system.
Preparation of standard stock and working standard solutions
10 mg of standard drug was taken in 50 ml standard volumetric flask and dissolved in the mobile phase and further diluted to 50 ml mark with mobile phase. The above stock solution was further diluted with mobile phase to get concentration ranging from 10µg/ml to 50µg/ml.
Chromatography and qualitative analysis of Cefetamet pivoxil
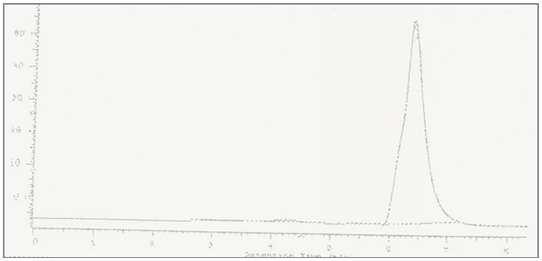
Working standard solution containing10µg/ml of Cefetamet pivoxil was injected into chromatograph composed of HYPERSIL C-18 column as stationary phase and separation of analyte was carried out using mobile phase composed of acetonitrile: water (80:20 v/v). The flow rate was with UV detection of 251 nm. The temperature of the column was ambient with average pressure was 55 psi. Chromatogram obtained was presented in [Figure 2] with retention time of. Hence retention time was used as one of the qualitative parameter of Cefetamet pivoxil as it shows selective separation at minute in selected stationary and mobile phase.
Extraction of cefetamet pivoxil and sample preparation
Twenty tablets were weighed accurately and finely powdered. The powder equivalent to 10 mg of Cefetamet pivoxil was taken in a 100 ml volumetric flask, mobile phase was added and kept in an ultrasonic bath for 10 min, and further diluted to the mark and filtered through membrane filter (nylon filters) of size 4.5 μ and used for analysis. The above sample solution was further diluted with mobile phase to get concentration ranging from 10µg/ml to 20 mg/ml.
Validation of developed HPLC method
Method was validated using performing analytical parameters such as specificity, selectivity, linearity, range, precision, ruggedness, robustness, detection limit, quantification limit and drug recovery.[10], [11]
Specificity and selectivity
Solution containing analyte and mobile phase was injected into chromatograph and chromatograms were obtained.
Linearity and range
Working standard solutions containing 10µg/ml, 20µg/ml, 30µg/ml, 40µg/ml and 50µg/ml of Cefetamet pivoxil was injected into chromatograph in triplicate chromatograms was obtained and integrated. Area of each injection was taken and calibration curve was plotted using concentration vs area of analyte obtained.
Precision
Method precision was performed by injecting the solutions containing Cefetamet pivoxil in six replicates on same day at different time intervals and on different days. Chromatograms were obtained and % RSD for area was calculated.
Robustness and ruggedness
Robustness was studied by changing the ratios of solvents used in mobile phase were as ruggedness was performed by different analyst, injecting six replicates of 10 µg/ml solution into chromatograph. Area was obtained from chromatograms and % RSD was calculated.
Drug recovery
A fixed amount of pre-analyzed sample was taken and standard drug was added at three different concentrations and each level was repeated for 3 times. The recovery was estimated to be more than99%.
Assay
20μl of standard and sample solutions were injected into an injector of liquid chromatography. The amount of Cefetamet pivoxil present per tablet was calculated by comparing the peak area, with that of the standard.
Results and Discussion
Development
RP-HPLC estimation was based on the reverse phase isocratic chromatographic mode. Separation of analyte was carried out by using C-18 column as stationary phase. Mobile phase composed of acetonitrile: water (80:20 v/v) was used at flow rate of 0.5mL/min. analysis was performed at ambient temperature. Retention time of Cefetamet pivoxil was found to be min. ([Figure 2]) optimized chromatographic parameters are presented in [Table 1].
|
Analyte: |
Cefetamet pivoxil |
|
Chromatographic mode |
Isocratic Reverse phase |
|
Chromatographic method |
HPLC |
|
Stationary phase |
Hypersil C-18 column |
|
Mobile phase |
Acetonitrile: water (80:20 v/v) |
|
Flow rate |
0.5 ml/min |
|
Detection wavelength |
251nm |
|
Retention time |
6.3 min |
Validation
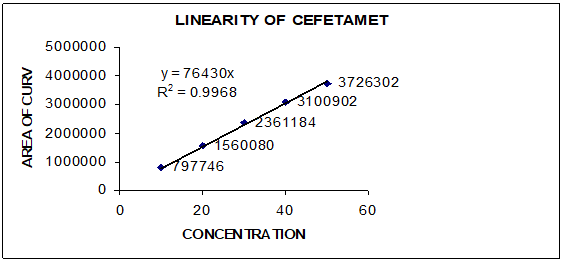
The method was found to be specific and selective as the chromatogram of blank sample showed no peak and interference of any area at the retention time of Cefetamet pivoxil in chromatograms obtained. Cefetamet pivoxil showed linear response between the concentrations of 10 – 50 µg/ml with regression coefficient of 0.9991. Standard calibration curve of Cefetamet pivoxil was showed in [Figure 2] and data of linearity and range was presented in [Table 2]. Method was found to be precise as the % RSD calculated for retention time and peak area in six replicates of injections in chromatograms was found to be less than 2% ([Table 3]) also the method was found to be rugged with % RSD of less than 2% for retention time and area obtained in chromatograms ([Table 4]). Detection limit and quantification limit of Cefetamet pivoxil was found to be 2.66µg/ml and 8.07µg/ml. Method was found to be accurate as the % recovery obtained at three different levels was found to be between 99 to 100% ([Table 5]). The assay results was found to be99.60%. The results of validation parameters were found to be well within the acceptance and presented in [Table 6].
|
Sr. No. |
Conc. |
*Retention time |
*Area |
Statistics |
Values |
|
1 |
10µg/ml |
6.36 min |
797746 |
r2 |
0.9968 |
|
2 |
20µg/ml |
6.36 min |
1560080 |
Slope |
76430 |
|
3 |
30µg/ml |
6.35 min |
2361184 |
LOD |
2.66µg/ml |
|
4 |
40µg/ml |
6.35 min |
3100902 |
LOQ |
8.07µg/ml |
|
5 |
50µg/ml |
6.35 min |
3726302 |
Range |
10-50µg/ml |
|
Precision |
Interday Precision |
Intraday Precision |
||
|
Replicates |
Peak area of 1 st hour |
Peak area of 6 th hour |
Peak area of day 1 |
Peak area of day 2 |
|
1 |
791740 |
781646 |
775541 |
781347 |
|
2 |
787849 |
787841 |
774944 |
781841 |
|
3 |
797943 |
797648 |
787649 |
797649 |
|
4 |
798648 |
795642 |
774642 |
795441 |
|
5 |
793441 |
783443 |
781444 |
783842 |
|
6 |
791845 |
791459 |
790442 |
791453 |
|
% RSD |
0.516% |
0.819% |
0.888% |
0.910% |
|
Replicates |
Retention time |
Peak area |
|
1 |
6.36 |
793026 |
|
2 |
6.36 |
798842 |
|
3 |
6.35 |
798642 |
|
4 |
6.35 |
800721 |
|
5 |
6.35 |
795420 |
|
6 |
6.36 |
799021 |
|
%RSD |
0.086% |
0.355% |
|
Recovery levels |
Amount of sample taken |
Amount of standard added |
Total amount |
Amount obtained |
% Recovery |
|
Level I |
10 mg |
5.0 mg |
15 mg |
14.90 mg |
99.33 |
|
Level II |
10 mg |
10 mg |
20 mg |
20.01 mg |
100.05 |
|
Level III |
10 mg |
15 mg |
25 mg |
24.98 mg |
99.92 |
|
Parameters |
Values obtained |
|
|
Specificity and selectivity |
No interference of any peak area at the retention time of analyte |
|
|
Linearity |
10-50 µg/ml |
|
|
Intra-day precision |
0.819% |
|
|
Inter-day precision |
0.910% |
|
|
Ruggedness |
0.355% |
|
|
Detection limit |
2.66µg/ml |
|
|
Quantification limit |
8.07µg/ml |
|
|
Recovery |
Level 1 |
99.33 % |
|
Level 2 |
100.05 % |
|
|
Level 3 |
99.92 % |
|
|
Assay |
99.60% |
Conclusion
RP-HPLC analytical method was developed for the estimation of Cefetamet pivoxil in bulk powder and validated as per the ICH guidelines. Developed and validated method was applied for the content estimation of analyte in its marketed formulation. All the results obtained was found to be within the acceptance limit of the guidelines also developed method was found to be simple, selective, specific, precise, accurate and rugged and can be used for the routine quality control of drug in bulk and dosage forms.
Authors Contributions
All authors have equally contributed in the research work.
Source of Funding
None.
Conflict of Interest
None.
Acknowledgment
The authors are thankful to Principal and Vice-Principal, KLE College of Pharmacy, Belgaum for their constant support and guidance given during the research project.
References
- . Find Drugs & Conditions. . 2022. [Google Scholar]
- . Today on Medscape. . 2022. [Google Scholar]
- Morsch LM, Bittencourt C, Souza M, Milano J. LC method for the analysis of Cefetamet pivoxil Hydrochloride in drug substance and powder for oral suspension. J Pharm Bio Ana. 2002;30(3):643-92. [Google Scholar]
- Vadia NH, Patel V. Spectrophotometric determination of Cefetamet pivoxil Pivoxil hydrochloride in bulk and in pharmaceutical formulation. Ind. J Pharm Sci. 2006;70(5):584-7. [Google Scholar]
- Mruthyunjayaswamy B, Hiremath B, Malipatal S, Appaluraja S. Spectrophotometric estimation of Cefetamet pivoxil in pharmaceutical formulation. Asian J Chem. 2007;19(5):3775-9. [Google Scholar]
- Sethi PD. Quantitative Analysis Of Drugs In Pharmaceutical Formulations. . 2001. [Google Scholar]
- Snyder LR, Kirkland JJ, Glajch JL. Practical HPLC development.. . 1997. [Google Scholar]
- Beckett AH, Stenlake J. Practical pharmaceutical chemistry-Part 2.. . 2002. [Google Scholar]
- Willard. Instrumental Methods Of Analysis. . 1986. [Google Scholar]
- Guidance I. Validation of high-performance liquid chromatography methods for pharmaceutical analysis: Understanding the differences and similarities between validation requirements of the US Food and Drug Administration, the US Pharmacopeia and the International Conference on Harmonization. J Chromatography A. 2005;987(1-2):57-66. [Google Scholar]
- . ICH Guidance, Validation of Analytical Procedures Methodology. International Conference on Harmonization, Q2B: Geneva; Nov, 2005. . . . [Google Scholar]
- Abstract
- Introduction
- Materials and Methods
- Drug samples
- Chemical and reagents
- Instruments and apparatus
- Methodology
- Stationary phase
- Preparation of mobile phase
- Selection of wavelength of detection
- Preparation of standard stock and working standard solutions
- Chromatography and qualitative analysis of Cefetamet pivoxil
- Extraction of cefetamet pivoxil and sample preparation
- Validation of developed HPLC method
- Specificity and selectivity
- Linearity and range
- Precision
- Robustness and ruggedness
- Drug recovery
- Assay
- Results and Discussion
- Validation
- Conclusion
- Authors Contributions
- Source of Funding
- Conflict of Interest
- Acknowledgment
- References
How to Cite This Article
Vancouver
Bhanusali M, Palled MS, Suryawanshi SS. Chromatographic estimation of cefetamet pivoxil in bulk and tablet dosage form: Development and validation approach [Internet]. Int J Pharm Chem Anal. 2022 [cited 2025 Oct 17];9(3):125-129. Available from: https://doi.org/10.18231/j.ijpca.2022.023
APA
Bhanusali, M., Palled, M. S., Suryawanshi, S. S. (2022). Chromatographic estimation of cefetamet pivoxil in bulk and tablet dosage form: Development and validation approach. Int J Pharm Chem Anal, 9(3), 125-129. https://doi.org/10.18231/j.ijpca.2022.023
MLA
Bhanusali, Mahesh, Palled, M. S., Suryawanshi, Shailendra S.. "Chromatographic estimation of cefetamet pivoxil in bulk and tablet dosage form: Development and validation approach." Int J Pharm Chem Anal, vol. 9, no. 3, 2022, pp. 125-129. https://doi.org/10.18231/j.ijpca.2022.023
Chicago
Bhanusali, M., Palled, M. S., Suryawanshi, S. S.. "Chromatographic estimation of cefetamet pivoxil in bulk and tablet dosage form: Development and validation approach." Int J Pharm Chem Anal 9, no. 3 (2022): 125-129. https://doi.org/10.18231/j.ijpca.2022.023
