- Visibility 734 Views
- Downloads 323 Downloads
- Permissions
- DOI 10.18231/j.ijpca.2021.029
-
CrossMark
- Citation
Immunomodulatory effect of Phyllanthus niruri
- Author Details:
-
Chandana G
-
Manasa R
-
Rajeshwari J
-
Shekhara Naik R
-
Mahesh Shivananjappa *
Abstract
Immunomodulators are being used to revive immune imbalance which helps in preventing and healing many diseases. Phyllanthus niruri (P. niruri) which is commonly called as stone breaker is widely distributed in tropical areas and known for its various ethnomedicinal and pharmacological effects. P. niruri is intensively being studied for its immunomodulating effects because of its wide-ranging uses in treating diseases related to immune system in indigenous medicine by scientifically studying and evaluating it in various clinical trials for the treatment of Pulmonary Tuberculosis, chronic Hepatitis- B, Varicella-Zoster and Vaginitis infection. In such diseases, effective immune system is essential for curing the disease and elimination of the pathogens. It has proved for its ability to modulate and activate the immune system through clinical studies.
Introduction
The human immune system is well organized comprising of many different immune cells like macrophages, neutrophils, T-lymphocytes, natural killer cells and various other specialized immune molecules like cytokines, antibodies and complement proteins that have evolved to mediate resistance against infections which is due to presence of active ingredients like bioactive phytochemicals which include lignans, flavonoids, terpenoids, alkaloids, polyphenols, coumarins, saponins, tannins and broad pharmacological actions.[1] The methanol extract of P. niruri was reported to modulate both innate and adaptive immune responses. Among the compounds abundantly identified in the plant, catechin, quercetin, and astragalin have been reported for their ability to regulate the immune system.[2], [3]
Human studies
Repandusinic acid inhibited human immune deficiency virus by type-1 reverse transcriptase (HIV-1-RT) with an ID50 value of 0.05mM. It showed 10-fold more sensitivity towards HIV-1-RT compared with human DNA polymerase-a, which is an indication of good selectivity in inhibiting HIV replication without harming normal cells of the body when they were exposed to extract. In addition, 4.5 mM of this compound inhibited 50% of HIV-1 induced giant cell formation. Also, at 2.5 mM it inhibited HIV-1 specific p24 antigen production in Clone H9 cell system. The compound showed strong inhibitory activity against HIV-1 protease, with an IC50 value of 12.5 mM.[4], [5]
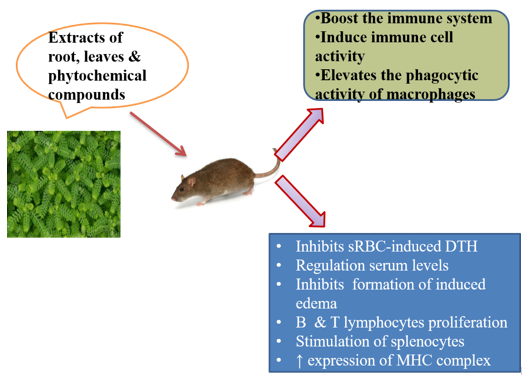
|
Model |
Extract |
Dose |
Parameter |
Result |
|
|
Female C57BL/6 Mice |
Aqueous extracts |
0, 12.5, 25, 50, 100 & 200 μg/ml |
FACS analysis |
Production of surface activation maker B and T lymphocyte proliferation Stimulation of naive splenocytes |
|
|
Transgenic OT-1 mice |
Aqueous extracts |
25, 50, and 100 microg/mL for 48 h |
BM/DCs |
Expression of MHC complex-ii and markers for dendritic cell maturation, activation & stimulation |
|
|
TB patients |
Aqueous extract |
Dose dependent |
Peripheral blood mononuclear cells (PBMCs) and macrophages were collected from active pulmonary TB patients |
Boosting the immune system Elevates the phagocytic activity of macrophages |
|
|
Swiss albino Mice |
Methanol extract |
200,400 mg/kg |
Total leucocytes count |
Inhibiting sRBC-induced DTH |
|
|
Swiss albino Mice |
Methanol extract |
200,400 mg/kg |
Total leucocytes count |
Regulated serum levels |
|
|
Musculous swiss male mice |
Spray-dried standardized extracts |
100, 200, 800, 1600 mg/kg |
Analytical balance |
Inhibition of carrageen induced edema & thioglycolate induced neutrophil migration |
|
|
Fishes |
Aqueous extracts |
0.002 to 20 mg/kg body weight |
Blood samples |
Enhance the efficacy of vaccines |
Invitro studies
Nworu and others found the effect of Aqueous extracts of P. niruri on spleen cells of female C57BL/6 mice (at a dose of 12.5-200 μg/ml for 18 h), there was increase in the expression of induced surface activation marker (CD69), B and T lymphocyte proliferation. The extract stimulated naive splenocyte by increasing the release of interferon gamma (IFN- γ) and interleukin-4 (IL-4) in a concentration dependent manner. Then various indices of activation and functions of murine bone marrow derived macrophages, such as phagocytosis, lysosomal enzyme activity and TNF-alpha release were significantly enhanced by pretreatment with P. niruri extract, which also modulated macrophage nitric oxide by release.[6] They also studied the effect of Aqueous extracts of P. niruri on female C57BL/6 mice and transgenic OT-1 mice (at a dose of 25-100 μg/ml for 48 h), there was an increase in the expression of major histocompatibility complex-II and markers for dendritic cell maturation (CD40), activation (CD83) and co-stimulation (CD86).[7] Putrid and coworkers found that the effect of Aqueous extracts of P. niruri on tuberculosis, patient there was an increase in nitric oxide, phagocytic activity and boosts the immune system in a dose dependent fashion, ultimately modulating the immune responses.[8]
Animal studies
Bonilla and oettgen found the effect of methanol extract of P. niruri on Swiss albino mice (at a dose of 200, 400 mg/kg) which helps in regulation of cellular immune responses by inhibiting sRBC induced DTH (Delayed-Type-Hypersensitivity) reaction. DTH is an important cell-mediated immune response and occupies the central role to protect the body against intracellular pathogens. This cellular response is primarily mediated by T helper type-1 cells which releases the cytokine IFN-γ. This cytokine causes the activation of macrophages and accounts for the symptoms of inflammation. Also, cytotoxic T cells and memory T cells are considered to be involved in DTH reaction[9], [13] Animal studies by Muthulakshmi and others found the effect of Aqueous extracts of P. niruri on Oreochromis mossambicus (at a dose of 0.02 to 20 mg/kg body weight) stimulating effects on neutrophil activation and the antibody response and later it can be used either as a routine feed supplement to activate the immune system of farmed fishes or as an adjuvant to enhance the efficacy of vaccines.[12] Eze and co-workers found the effect of methanol extract of P. niruri on Swiss albino mice (at a dose of 200, 400mg/kg) and found to regulate the serum levels of primary and secondary antibodies.[10] Porto and others worked on spray-dried standardized extracts with varied concentrations of 100, 200, 800, 1600 mg/kg have found to inhibit carrageen induced edema and thioglycolate induced neutrophil migration in musculus Swiss male mice.[11]
Conclusion
Altogether, all of the studies depicted P. niruri’s clinical efficacy as an immunomodulator through the activation and augmentation of the cellular immune system. Specifically, P. niruri activates neutrophils, macrophages or monocytes, and T and B lymphocytes. Activated phagocytic process by the neutrophils suggests an acceleration of the active eradication process, particularly of the extracellular pathogens, such as invading viruses, microbes, or fungi and their removal from our bodies. Further, the modulation of cytokine secretion by P. niruri treatment, as observed in various clinical studies, such as stimulation to IFN-γ, TNF-α, IL-4, IL-6 strongly indicates that P. niruri influences our body’s defense reaction involving cellular immune system against foreign pathogens.
Source of Funding
None.
Conflict of Interest
None.
References
- Tjandrawinata RR, Susanto LW, Nofiarny D. The use of Phyllanthus niruri L. as an immunomodulator for the treatment of infectious diseases in clinical settings. Asian Pac J Trop Dis. 2017;7(3):132-40. [Google Scholar] [Crossref]
- Bagalkotkar G, Sagineedu SR, Saad MS, Stanslas J. Phytochemicals from Phyllanthus niruri Linn. and their pharmacological properties: a review. J Pharm Pharmacol. 2006;58(12):1559-70. [Google Scholar] [Crossref]
- El-Aziz A, Mohamed TA, Pasha RH, Aziz HF. Catechin protects against oxidative stress and inflammatory-mediated cardiotoxicity in adriamycin-treated rats. Clin Exp Med . 2012;12(4):233-40. [Google Scholar] [Crossref]
- Ogata T, Higuchi H, Mochida S, Matsumoto H, Kato A, Endo T. HIV-1 reverse transcriptase inhibitor from Phyllanthus niruri. AIDS Res Hum Retroviruses. 1992;8(11):1937-44. [Google Scholar] [Crossref]
- Xu HX, Wan M, Dong H, But PPH, Foo LY. Inhibitory activity of flavonoids and tannins against HIV-1 protease. Biol Pharm Bull. 2000;23(9):1072-6. [Google Scholar] [Crossref]
- Lordelo EDR, Fonseca AL, Araújo MLVD. Responsividade do ambiente de desenvolvimento: crenças e práticas como sistema cultural de criação de filhos. . Psicologia: Reflexão Crítica. 2000;13(1):73-80. [Google Scholar]
- Eze CO. Immunomodulatory activities of methanol extract of the whole aerial part of Phyllantus niruri L. J Pharmacogn Phytother. 2014;6(4):41-6. [Google Scholar] [Crossref]
- Bonilla FA, Oettgen HC. Adaptive immunity. J Allergy Clin Immunol. 2010;125(2):33-40. [Google Scholar]
- Nworu CS, Akah PA, Okoye FBC, Proksch P, Esimone CO. The effects of Phyllanthus niruri aqueous extract on the activation of murine lymphocytes and bone marrow-derived macrophages. Immunol Investig. 2010;39(3):245-67. [Google Scholar] [Crossref]
- Putri DU, Rintiswati N, Soesatyo MH, Haryana SM. Immune modulation properties of herbal plant leaves: Phyllanthus niruri aqueous extract on immune cells of tuberculosis patient-in vitro study. Nat Product Res. 2018;32(4):463-7. [Google Scholar] [Crossref]
- Nworu CS, Akah PA, Okoye FB, Esimone CO. Aqueous extract of Phyllanthus niruri (Euphorbiaceae) enhances the phenotypic and functional maturation of bone marrow-derived dendritic cells and their antigen-presentation function. Immunopharmacol Immunotoxicol. 2010;32(3):393-401. [Google Scholar] [Crossref]
- Muthulakshmi M, Subramani PA, Michael RD. Immunostimulatory effect of the aqueous leaf extract of Phyllanthus niruri on the specific and nonspecific immune responses of Oreochromis mossambicus Peters. Iranian J Vet Res. 0200;17(3):200-2. [Google Scholar]
- Chen JJ, Chung CY, Hwang TL, Chen JF. Amides and benzenoids from Zanthoxylum ailanthoides with inhibitory activity on superoxide generation and elastase release by neutrophils. J Nat Prod. 2009;72(1):107-11. [Google Scholar] [Crossref]
How to Cite This Article
Vancouver
G C, R M, J R, R SN, Shivananjappa M. Immunomodulatory effect of Phyllanthus niruri [Internet]. Int J Pharm Chem Anal. 2021 [cited 2025 Nov 05];8(4):152-154. Available from: https://doi.org/10.18231/j.ijpca.2021.029
APA
G, C., R, M., J, R., R, S. N., Shivananjappa, M. (2021). Immunomodulatory effect of Phyllanthus niruri. Int J Pharm Chem Anal, 8(4), 152-154. https://doi.org/10.18231/j.ijpca.2021.029
MLA
G, Chandana, R, Manasa, J, Rajeshwari, R, Shekhara Naik, Shivananjappa, Mahesh. "Immunomodulatory effect of Phyllanthus niruri." Int J Pharm Chem Anal, vol. 8, no. 4, 2021, pp. 152-154. https://doi.org/10.18231/j.ijpca.2021.029
Chicago
G, C., R, M., J, R., R, S. N., Shivananjappa, M.. "Immunomodulatory effect of Phyllanthus niruri." Int J Pharm Chem Anal 8, no. 4 (2021): 152-154. https://doi.org/10.18231/j.ijpca.2021.029
