- Visibility 118 Views
- Downloads 15 Downloads
- Permissions
- DOI 10.18231/j.ijpca.2021.023
-
CrossMark
- Citation
Fabrication of zero-valent iron nanoparticles by green and chemical reduction methods: Application in the field of antibacterial activities for medicinal point of view
- Author Details:
-
Manish Srivastava *
-
Preeti Tomer
-
Anamika Srivastava
-
Swapnil Sharma
Abstract
In the area of life sciences, iron nanoparticles (Fe NPs) have many applications. In this paper, the unique properties of iron nanoparticles as antimicrobials are studied. In this study, nanoparticles of iron have been fabricated by green and chemical reduction method. With the help of FESEM analysis and Zeta size analysis, the usual value of nanoparticles was found to be 10-30 nm in size. Furthermore, the prepared nanoparticles were examined for antibacterial perspective aligned with gram-positive and negative strains namely Staphylococcus aureus & Bacillus subtilis and Escherichia coli, using agar plate method and IC50 was also estimated using tube dilution assay.
Introduction
Nanotechnology is an extremely convincing technology with its approaching medical properties in early disease detection and treatment. At present, there is a growing concern in the research of nano-metals as these signify an intermediate dimension between atoms/molecules and bulk particles.[1] Among metal nanoparticles, iron oxide has received more attention because of its variety of scientific and technological applications such as antimicrobial activity[2], biosensor[3], food preservation[4], ferrofluids, magnetic resonance imaging, magnetic refrigeration, magnetic storage media, cell sorting, targeted drug delivery, and hyperthermia cancer treatments.[5], [6], [7]
The size of nanoparticles makes them an attractive applicant for in vivo and in vitro biomedical research. The incorporation of nanoparticles in the medical field has led to their special use in the field of sensing, drug delivery, imaging, and artificial implants. An interesting new approach for their investigation in the medical field is their application in antimicrobial activities that aim at drug-resistant and highly pathogenic microbes.[8]
There is also an increasing interest in the application of Iron and other metal nanoparticles in blood platelet sanitization. Ag+ ions may slow down some imperative transport processes such as succinct and phosphate uptake, by contacting the cellular oxidation process in the respiratory string. The high absorption of Ag+ not only causes toxicity but may also lead to electrolyte imbalances in patients.[9]
In recent research, it has been shown that the better antibacterial properties of iron nanoparticles (Fe NPs) are dependent upon the high surface area and high volume of nanoparticles that allow proficient bacterial disinfection.[10], [11] Iron nanoparticles (Fe NPs) have a larger surface area compared to silver so that they possess better antimicrobial properties as compared to Ag+ ions.[12], [13], [14], [15] Despite this, in an aqueous solution zero-valent iron nanoparticles (Fe NPs) can inactivate E. coli. Which increases the interest of iron nanoparticles in antimicrobial activity. In the deficiency of oxygen, iron nanoparticles (Fe NPs) show high antimicrobial activity analogous to that of silver nanoparticles (Ag NPs). The strong antibacterial activity of iron nanoparticles (Fe NPs) implies that Fe NPs can serve as a cost-effective biocide for many of the applications in which silver is being used.[16] In the case of antibacterial activity, iron nanoparticles (Fe NPs) involve the release of Fe ions on the biological obligatory site that hold upon to the surface of the targeted atom and developed a bacterial interaction with the NPs that starts from the surface layer.[17], [18], [19]
Materials and Methods
Green method
Fabrication of Fe NPs using S oleracea aqueous leaf extract
Fe NPs were fabricated by using fresh leaves of S. oleracea extract as a reducing agent. For the fabrication of Fe NPs, fresh leaves of S. oleracea were washed, dried at room temperature, and cut into small pieces. Dried-out leaves are crushed into powdered form and from which 4g was mixed in 50 ml of double distilled water and then boiled for 15 min at 100oC. Filter the extract and stored it at 40 C. Furthermore, 10ml of S. oleracea extract was added into 10ml aqueous solution of 1.0 mM ferric chloride. The mixture was boiled for about half an hour and after which black color was observed, representing the fabrication of Fe NPs.[20]
Chemical reduction method
Fabrication of Fe NPs using NaBH4 as reducing agent
In a three-neck, round-bottom flask 0.1M FeCl3.6H2O (4.1703 g) and 0.05M EDTA (3.7224 g) were mixed in 100 ml Milli-Q water by using appropriate mixing. Then the solution of NaBH4 (0.75M NaBH4 (2.837 g) in 100 ml Milli-Q water) was added dropwise into the above solution. Slowly the solution changed to black color.[21], [22], [23]
Results and Discussion
Characterization of Fe NPs
Analysis by ultraviolet-Visible spectra
An ultraviolet-Visible spectrum is an effectual technique that illustrates the development of varieties of metals in the creation of colloidal M NPs. As exposed in Fig. 1a the optical absorbance Plasmon band of the Fe NPs using S. oleracea extract in the colloidal suspension is about 267 & 229. In the case of optical absorbance Plasmon band of Fe NPs using chelating Agents (EDTA) Fig-1b in the colloidal suspension is about 273 & 224. In both cases, the black color appeared due to the formation of Fe NPs.

Zeta size measurement
The zeta potential value (21.9) shows the stability of Fe NPs using S. oleracea extract and confirms the size of Fe NPs particles to be 80.37(d. nm). On the other hand, the zeta potential value (-21.2) of Fe NPs using classical reduction method with EDTA and found the size of Fe NPs to be 51.37 (d. nm) (Fig- 2: A&B).

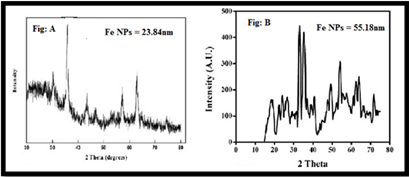
XRD of Fe NPs by chemical reduction and green method
The aqueous solution of Fe NPs was frequently centrifuged at 10,000 rpm for 30 min, redispersed with distilled water, and lyophilized to get pure Fe NPs pellets. The Debye-Scherer principle has been used to evaluate the standard particle size that could be understood as:
D = 0.9λ/ β Cosθ
An average dimension of Fe NPs was found in the green method is 55.18 nm and in the chemical reduction method 23.84 nm by XRD methods. (Fig-3: A&B)
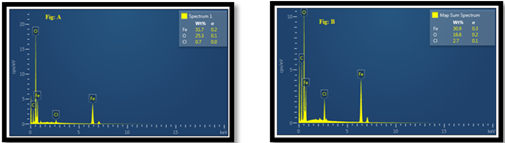
EDX of Fe NPs by chemical reduction and green method
EDX is Energy Dispersive X-Rays (aka EDAX - energy dispersive x-ray analysis) and is reliant on the atomic mass of the elements being detected. In the fabrication of Fe NPs in the chemical reduction method the wt% value of Fe is 31.7, O is 25.3 and Cl is 0.7 and in the case of the green method the wt% value of Fe is 30.9, O is 16.2 and Cl is 2.7. The peak of Fe presents in the spectrum shows the fabrication of Fe NPs.
FE SEM analysis of Fe NPs
The FE SEM image shows that the size of indusial Fe NPs was found to be around 50-60 nm using S. oleracea extract confirms the size of Fe NPs particles to be 51.22 (d. nm). On the other hand, the Fe NPs using the classical reduction method with EDTA was found to be 47.97(d. nm) (Fig-5)

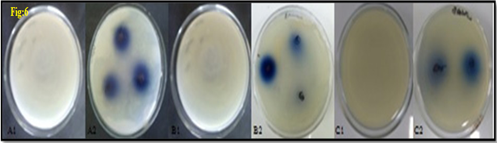
In-vitro antibacterial studies of nanoparticles
The iron nanoparticles produced by the liquid phase reduction technique were subjected to the in-vitro antibacterial activity of iron nanoparticles aligned with gram-negative (E. coli) and gram-positive (S. aureus and B. subtilis) respectively. Iron nanoparticles showed excellent and dose-dependent activity when compared to a standard antibiotic. The effects obtained after 24h incubation are presented in table 1 and fig6. Test compound the highest region of prohibition at 500μg/mL was 21 ± 0.64 mm as compared to standard ampicillin.
|
Test compound |
Zone of inhibition (mm) |
||
|
E. coli |
S. aureus |
B. subtilis |
|
|
Ampicillin (Standard) (80 μg/mL) |
20 ± 0.56 |
15 ± 0.23 |
18 ± 0.85 |
|
200μg/mL |
12 ± 0.54 |
13 ± 0.47 |
14 ± 0.76 |
|
300μg/mL |
16 ± 0.36 |
15 ± 0.22 |
15 ± 0.51 |
|
400μg/mL |
18 ± 0.76 |
17 ± 0.61 |
17 ± 0.33 |
|
500μg/mL |
19 ± 0.47 |
20 ± 0.72 |
21 ± 0.64 |
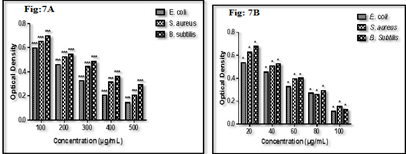
Tube dilution assay
In tube dilution assay, prepared nanoparticles showed significant antibacterial activities against targeted bacterial strains. IC50 of test compound was found to be 350 μg/mL, 300 μg/mL, and 400 μg/mL against bacterial strains compared with IC50of standard ampicillin was found to be 40 μg/mL,50 μg/mL, and 50 μg/mL against pathogenic E. coli, S. aureus and B. subtilis respectively (Fig. 2a &2b). Test compounds have shown better activities against B. subtilis and it was observed that it showed maximum ZOI and minimum IC50 (300 μg/mL) in both in vitro models. It indicates that it can be useful in the management of various B. subtilis mediated infections. The results of biological activity are revealed that iron nanoparticles found active against both strains but slightly better against gram-positive strains. The findings of the study were found in agreement with previously published reports (Behera et al. 2012).
Conclusion
Today, iron nanoparticles’ production with the help of plant materials or in an eco-friendly manner is considered attractive. The attrition of metallic ions into base metals by the biological method is a rapid and more efficient method than conventional methods. This method is eco-friendly and can be conducted willingly at room temperature and is also amplified easily. The reducing agents drawn in contain various water-soluble metabolites and coenzymes. The reward of holding green methods for nanoparticle amalgamation has increased the demand and the curiosity of analyzers to examine the procedures for uptake of metal ions and biological reducing methods by plants. Moreover, green synthesis generates nanoparticles that are highly stable and have narrow size distribution with high discrepancy.
Source of Funding
None.
Conflict of Interest
None.
References
- Iglesias S, Rivas J, Isidro LML, Quintela MAL. Synthesis of Silver-Coated Magnetic Nanoparticles. J Non-Crystal Solids. 2007;353(8-10):829-31. [Google Scholar] [Crossref]
- Berry CC, Curtis ASG. Functionalisation of Magnetic Nanoparticles for Applications in Biomedicine. J Phys D: ApplPhysics. 2003;36(13):198-206. [Google Scholar]
- Babes L, Denizot B, Tanguy G, Jeune JJL, Jallet P. Synthesis of Iron Oxide Nanoparticles Used as MRI Contrast Agents: A Parametric Study. J Colloid Interface Sci. 1999;212(2):474-82. [Google Scholar] [Crossref]
- Chan DCF, Kirpotin DB, Bunn PA. Synthesis and Evaluation of Colloidal Magnetic Iron Oxides for the Site-Specific Radio Frequency Induced Hyperthermia of Cancer. J Magnetism Magn Mater. 1993;122(1):374-8. [Google Scholar] [Crossref]
- B-Tan RGH, Engelshoven JMA, Greve JWM. Hepatic Adenoma and Focal Nodular Hyperplasia: MR Findings with Superparamagnetic Iron Oxide- enhanced MRI. Clin Imaging. 1998;22(3):211-5. [Google Scholar] [Crossref]
- Gupta AK, Gupta M. Synthesis and Surface Engineering of Iron Oxide Nanoparticles for Biomedical Applications. Biomaterials. 2005;26(18):3995-4021. [Google Scholar] [Crossref]
- Lida H, Takayanagi K, Nakanishi T, Osaka T. Synthesis of Fe3O4 Nanoparticles with Various Sizes and Magnetic Properties by Controlled Hydrolysis. J Colloid Interface Sci. 2007;314(1):274-80. [Google Scholar] [Crossref]
- Samia ACS, Dayal S, Burda C. Quantum dot-based energy transfer: Perspectives and potential for applications in photodynamic therapy. Photochemistry Photobiology. 2006;82(3):617-25. [Google Scholar] [Crossref]
- Lee HJ, Yeo SY, Jeong SH. Antibacterial effect of nanosized silver colloidal solution on textile fabrics. J Mater Sci. 2003;38:2199-2204. [Google Scholar]
- Morones JR, Elechiguerra JL, Camacho K, Holt JB, Kouri JT, Ramírez MJ. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16:2346-53. [Google Scholar]
- Baker C, Pradhan A, Pakstis L, Pochan DJ, Shah SI. Synthesis and antibacterial properties of silver nanoparticles. J Nanosci Nanotechnol. 2005;5(2):244-9. [Google Scholar] [Crossref]
- Tolaymat TM, Badawy AM, Genaidy K, Scheckel TP, Luxton M, Suidan. An evidence-based environmental perspective of manufactured silver nanoparticle in syntheses and applications: a systematic review and critical appraisal of peer-reviewed scientific papers. Sci Total Environ. 2010;408(5):999-1006. [Google Scholar] [Crossref]
- Lee D, Cohen RE, Rubner M. Antibacterial Properties of Ag Nanoparticle Loaded Multilayers and Formation of Magnetically Directed Antibacterial Microparticles. Langmuir. 2005;21(21):9651-9. [Google Scholar] [Crossref]
- Yoon KY, Byeon JH, Park JH, Hwang J. Susceptibility constants of Escherichia coli and Bacillus subtilis to silver and copper nanoparticles. Sci Total Environ. 2007;373(2-3):572-5. [Google Scholar] [Crossref]
- Chopra IJ. The increasing use of silver-based products as antimicrobial agents: a useful development or a cause for concern?. J Antimicrob Chemother. 2007;59(4):587-90. [Google Scholar] [Crossref]
- Rana S, Kalaichelvan P. Anti-bacterial activities of metal nanoparticles. Adv Biotech. 2011;2(11):21-3. [Google Scholar]
- Egger S, Lehmann RP, Height M, Loessner MJ, Schuppler M. Antimicrobial Properties of a Novel Silver-Silica Nanocomposite Material. Appl Environ Microbiol. 2009;75(9):2973-6. [Google Scholar] [Crossref]
- Panacek A, Kvıtek L, Prucek R, Kolar M, Vecerova R, Pizurova N. Silver colloid nanoparticles: synthesis, characterization, and their antibacterial activity. J Phys Chem B. 2006;110(33):16248-53. [Google Scholar] [Crossref]
- Sharma VK, Yngard RA, Lin Y. . Adv Colloid Interface Sci. 2009;145:83-96. [Google Scholar]
- Eftekhari KK, Mehraj S, Tarigopula MP. . Int J Plant. 2015;5(1). [Google Scholar]
- BM, Mandal K, Kesarla KMK, Kumar PSS, Allabaksh B. Preparation of Stable Zero Valent Iron Nanoparticles using Different Chelating Agents. J Chem Pharm Res. 2010;2(5):67-74. [Google Scholar]
- Pandey B, Ghimire P, Agrawal VP. Studies on the antibacterial activity of actinomycetes isolated from Khumbu region of Mount Everest. J App Microbiol. 2004;20:45-54. [Google Scholar]
- Gebreyohannes G, Moges F, Sahile S, Raja N. . Asian Pac J Trop Biomed. ;2013(6):60092-3. [Google Scholar] [Crossref]
- Abstract
- Introduction
- Materials and Methods
- Results and Discussion
- Characterization of Fe NPs
- Analysis by ultraviolet-Visible spectra
- Zeta size measurement
- XRD of Fe NPs by chemical reduction and green method
- EDX of Fe NPs by chemical reduction and green method
- FE SEM analysis of Fe NPs
- In-vitro antibacterial studies of nanoparticles
- Conclusion
- Source of Funding
- Conflict of Interest
- References
How to Cite This Article
Vancouver
Srivastava M, Tomer P, Srivastava A, Sharma S. Fabrication of zero-valent iron nanoparticles by green and chemical reduction methods: Application in the field of antibacterial activities for medicinal point of view [Internet]. Int J Pharm Chem Anal. 2021 [cited 2025 Sep 22];8(3):118-122. Available from: https://doi.org/10.18231/j.ijpca.2021.023
APA
Srivastava, M., Tomer, P., Srivastava, A., Sharma, S. (2021). Fabrication of zero-valent iron nanoparticles by green and chemical reduction methods: Application in the field of antibacterial activities for medicinal point of view. Int J Pharm Chem Anal, 8(3), 118-122. https://doi.org/10.18231/j.ijpca.2021.023
MLA
Srivastava, Manish, Tomer, Preeti, Srivastava, Anamika, Sharma, Swapnil. "Fabrication of zero-valent iron nanoparticles by green and chemical reduction methods: Application in the field of antibacterial activities for medicinal point of view." Int J Pharm Chem Anal, vol. 8, no. 3, 2021, pp. 118-122. https://doi.org/10.18231/j.ijpca.2021.023
Chicago
Srivastava, M., Tomer, P., Srivastava, A., Sharma, S.. "Fabrication of zero-valent iron nanoparticles by green and chemical reduction methods: Application in the field of antibacterial activities for medicinal point of view." Int J Pharm Chem Anal 8, no. 3 (2021): 118-122. https://doi.org/10.18231/j.ijpca.2021.023
