- Visibility 255 Views
- Downloads 138 Downloads
- Permissions
- DOI 10.18231/j.ijpca.2024.025
-
CrossMark
- Citation
Quantitative estimation of fenticonazole nitrate by zero-order derivative area under curve spectrophotometric methods in bulk and in-capsule dosage form
- Author Details:
-
Ashish Gorle *
-
Pritam Jain
-
Pankaj Nerkar
-
Nitin Haswani
-
Saurabh Chordiya
-
Saipudeen H. Kommakayam
Abstract
The purpose of this study is to establish Zero-order UV-Spectrophotometric - absorbance and Zero Order- Area under curve (AUC) methods for estimation of Fenticonazole Nitrate in bulk and vaginal capsules. Fenticonazole Nitrate is an antifungal drug and it is completely insoluble in water. Methanol was used as solvent for solubilization of Fenticonazole Nitrate. Maximum absorption for Fenticonazole Nitrate was found to be at wavelength 253 nm when dissolved in Methanol. The methods are based upon measurement of absorbance at 253nm and integration of area under curve for analysis of Fenticonazole Nitrate in the wavelength range of 242-262 nm. The drug followed linearity in the concentration range of 5 - 30µg/mL with correlation coefficient value r2> 0.99 for both methods. The proposed methods were validated for accuracy (% recovery), precision, repeatability and ruggedness, as per ICH guidelines. The proposed methods were applied for qualitative and quantitative estimation of Fenticonazole Nitrate in vaginal capsules and results were found in good agreement with the label claimed. Developed methods can be used for routine analysis of Fenticonazole Nitrate in bulk and Vaginal Capsules.
Introduction
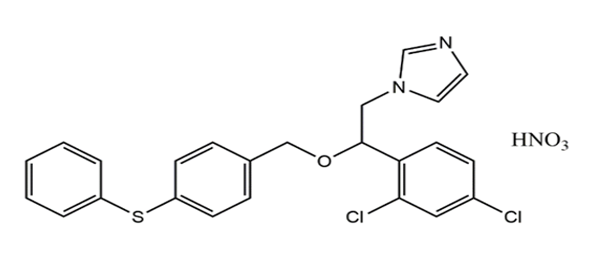
Fenticonazole Nitrate (FTZ) is an Imidazole antifungal drug used in the treatment of vulvovaginal candidiasis. It is active against the range of organism including dermatophyte pathogens, Malassezia furfur, and Candida albicans.[1] The interesting mechanism of action of Fenticonazole is inhibition of the secretion of protease acid by Candida albicans, damage to the cytoplasmic membrane; and by blocking cytochrome oxidases and peroxidises [2] Fenticonazole Nitrate chemically is 1-[2(2,4-dichlorophenyl)-2 {[4 (phenyl sulfanyl) phenyl]methoxy}ethyl]1H-imidazole. The chemical structure of Fenticonazole Nitrate is shown in [Figure 1] Fenticonazole is official reported in EP with their characteristics, Identification test, Assay and Impurity detection.[3] Some literature of Fenticonazole is also exist in Merck index and Martindale.[4], [5] The information of related substance with their identification test are reported in BP.[6] In literature, few liquid chromatography procedures have been reported for the analysis of Fenticonazole Nitrate.[7], [8], [9], [10], [11] The present UV-Spectrophotometric methods were designed for estimation of Fenticonazole Nitrate using Methanol as a solvent. The current works emphasize simple, precise, sensitive and effective UV Spectroscopy method for estimation of Fenticonazole Nitrate in bulk and in-Vaginal capsules. The method was validated as per ICH guidelines.
Chemical and Reagents
Fenticonazole Nitrate was gifted by Akum Lifescience ltd. Haridwari. All Chemicals and reagents are AR Grade and purchased from Rankem Chemicals Pvt. Ltd.Mumbai and Vaginal Capsule (Fentin 600mg) was purchased from local market.
Instrument
Spectrophotometer: UV-2450 Shimadzu, Japan
Software: UV Probe 2.21
Sample cell: 1cm quartz cuvette
Lamp: Deuterium Lamp Wavelength range 200 – 400nm
Spectral Slit width: 1.0nm
Weighing Balance: Shimadzu AUX-120
Experimental
Preliminary solubility studies and selection of solvent
Solubility of FTZ was found in Methanol and Dimethylformamide (DMF). The Fenticonazole Nitrate is completely soluble in methanol. So for further study Methanol is used as a solvent.
Preparation of stock standard solution
Stock standard solution of FTZ was prepared by dissolving accurately weighed 10mg of FTZ into 100ml of Methanol, to obtain concentration of 100µg/mL. sonicated for 5 min. The working standards were prepared by dilution of the stock standard solution.
Determination of λ max and calibration curve
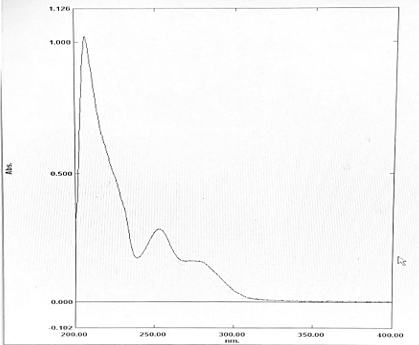
A fixed volume of 1.0mL of FTZ from stock solution was transferred to 10mL volumetric flask, diluted to mark with Methanol to obtain concentration of 10µg/mL. The resultant solution was scanned in UV range (400-200nm) in 1.0cm cell against solvent blank. The spectrum showed an absorption maximum at 253nm.
In Method I absorbance at 253nm was considered for analysis while for Method II two wavelengths 242-262 nm were selected for determination of Area under Curve [AUC]. Optical Characteristics of FTZ presented in.[Table 1]. Zero order UV-spectrum showing maximum absorbance and AUC are shown in.[Figure 2]
|
Parameters |
Method 1 |
Method 2 (AUC) |
|
Linearity Range |
5-30 |
5-30 |
|
Wavelength / AUC Range |
253 |
242-262 |
|
Slope |
0.025 |
0.106 |
|
Intercept |
0.028 |
0.030 |
|
Correlation Coefficient |
0.999 |
0.998 |
|
LOD (µg) |
0.311787 |
0.32600 |
|
LOQ (µg) |
0.944811 |
0.987891 |
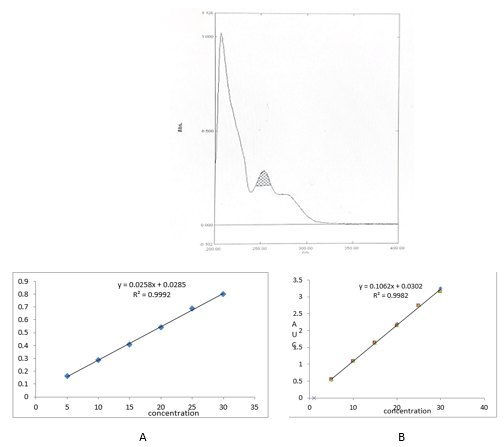
An appropriate volume of stock solution in the range of 0.5–3.0mL were transferred in series of 10mL volumetric flask, volume was made up to 10mL with Methanol to get concentration of 5–30µg/mL and absorbance was measured at 253nm (method I) and Zero order- AUC was recorded in between the wavelength range of 242-262 nm (Method II) against the blank. Calibration curves were prepared by plotting concentration versus absorbance and AUC as shown in.[Figure 3]
Analysis of Capsule formulation
Ten capsules were accurately weighed and average weight determined, an amount of power drug equivalent to 10 mg of Fenticonazole Nitrate was transfer into 100 mL volumetric flask containing 100 ML of Methanol, sonicated for 5 min. The volume was made up to the mark with same solvent and filtered through 0.45µm Whatman filter paper. A suitable volume of solution was further diluted with Methanol to obtain concentration 20µg/mL of Fenticonazole Nitrate for tablet assay. These sample solutions were scanned at selected wavelengths in Method I and Method-II and the results were obtained. From the respected linear regression equations, the concentrations were determined. The procedure was repeated for six times and the results are shown in.[Table 2]
|
|
Label claim(mg) |
Conc (µg/mL) |
%Amount found n=6 |
±SD |
±RSD |
|
Method 1 |
600 |
20 |
100.56 |
0.5428 |
0.5406 |
|
Method 2 |
600 |
20 |
100.06 |
0.1855 |
0.1853 |
|
%Value |
Initial Amount (µg/mL) |
Ammount Added (µg/mL) |
Method 1 |
|
Method 2 |
|
|
|
|
|
%Recovery |
%RSD |
%Recovery |
%RSD |
|
50% |
10 |
5 |
100.8 |
1.1223 |
100.37 |
1.8608 |
|
100% |
10 |
10 |
101 |
1.6602 |
101.32 |
1.4484 |
|
150% |
10 |
15 |
100.93 |
0.5635 |
99.935 |
0.089 |
|
Concentation (µg/mL) |
Intra-Day |
Inter-Day |
|||
|
Method 1 |
Method 2 |
%RSD |
%RSD |
||
|
|
|
Method 1 |
Method 2 |
Method 1 |
Method 2 |
|
15 |
15 |
1.170 |
1.291 |
0.9854 |
1.2553 |
|
20 |
20 |
0.6587 |
0.2708 |
1.5568 |
1.2068 |
|
25 |
25 |
1.4253 |
1.3544 |
1.2564 |
1.3676 |
Validation
The method was validated for accuracy, precision, and ruggedness according to ICH guidelines.
Accuracy/Recovery Studies
To estimate the accuracy of both the proposed methods, recovery studies were executed out at 50, 100 and 150% of the test concentration as per ICH guidelines. To the pre-analyzed sample solution (10µg/ml), a known amount of drug standard was added at 50, 100 and 150% and solutions were reanalyzed by proposed methods. The experiments were performed three times at each level for each method. The results of the recovery studies are reported in the table below.
Precision
Precision of the methods were studied as intra-day, inter-day variations and repeatability. Precision was determined by analyzing the concentration of 15, 20, and 25µg/ml. To determine the degree of repeatability of the methods, statistical evaluation was carried out, and the results are reported in the table below.
Repeatability
Repeatability was determined by analyzing Fenticonazole concentration of 20µg/mL for six times and results are reported in below table.
|
Method |
Concentration (µg/mL) n=6 |
%Amount Found |
%RSD |
|
Method 1 |
20 |
98.7 |
1.7277 |
|
Method 2 |
20 |
99.83 |
1.2410 |
Ruggedness
The ruggedness of the proposed method was determined by analysis of aliquots from homogenous slot by two analyst using same operational and environmental conditions and the results are reported in below table.
|
Method |
Amount taken (µg/mL) n=3 |
%Amount found |
|
|
|
|
Analyst 1 |
Analyst 2 |
|
Method 1 |
20 |
99.60 |
98.466 |
|
Method 2 |
20 |
98.883 |
98.396 |
Sensitivity
The sensitivity of proposed methods was estimated in terms of estimating Detection Limit (DL) and Quantitation Limit (QL) which were calculated using formulae “DL = 3.3 ×N/B,” and “QL = 10 × N/B” where “N” is average standard deviation of the absorbance or peak areas of the Fenticonazole (n = 3), taken as a measure of noise, and “B” is the slope of the corresponding calibration curve.
Results and Discussion
In the present investigation, Methanol is used to enhance the aqueous solubility of poorly water-soluble drugs Fenticonazole Nitrate in bulk and in vaginal capsules. By selecting proper solvent. For the solubility studies, different solvent was tried but optimum solubility was achieved in Methanol. In Method I and II, linearity of Fenticonazole Nitrate was found to be in the range of 05 - 30µg/mL, with correlation coefficient (r2 > 0.99). Marketed brand of Vaginal capsules were analyzed. The amounts of Fenticonazole Nitrate determined by ‘Method I’ and Method II was found to be 100.56% and 100.06, respectively. In both these methods, precision was studied as repeatability, inter and intra-day variations at three different concentrations of Fenticonazole Nitrate and % RSD was found to be less than 2. The accuracy of method was determined by calculating mean percentage recovery. It was determined at 50, 100 and 150% level and % recovery was found to be in the range 100.80% - 100.93% and 100.37% – 99.94% for method I and II respectively. The ruggedness of the methods was studied by two different analysts using the same operational and environmental conditions and % RSD found to be less than 2. DL and QL were found to be 0.3117µg/ml and 0.9448 µg/ml, respectively for Method I and DL and QL were found to be 0.3260µg/ml and 0.9878µg/ml respectively for Method II indicating adequate sensitivity of the methods.
Conclusion
The proposed methods for analysis of Fenticonazole Nitrate in pharmaceutical formulations are ecofriendly; simple, precise and rapid so can be employed for routine analysis. From the proposed methods, we conclude that there is a good scope for other poorly water-soluble drugs to get solubilized by using organic solvents.
Source of Funding
None.
Conflict of Interest
Authors declare that there is no direct or indirect conflict of interest with this research work.
References
- Fernández-Alba J, Valle-Gay A, Dibildox M, Vargas JA, González J, García M, et al. Fenticonazole nitrate for treatment of vulvovaginitis: efficacy, safety, and tolerability of 1-gram ovules, administered as ultra-short 2-day regimen. Journal of chemotherapy. 2004;16(2):179-86. [Google Scholar]
- Veraldi S, Milani R. Topical fenticonazole in dermatology and gynaecology. Drugs. 2008;68(15):2183-94. [Google Scholar]
- . European pharmacopeia Department of Health. . 2005. [Google Scholar]
- . . The Merk Index. 1986;29. [Google Scholar]
- Martindale T, Sean S. . Martindale: The Complete Drug Reference. . [Google Scholar]
- Pharmacopeia B. The Introduction General Notice Monography Medicinal and Pharmaceutical Substance (A-1). . 2005. [Google Scholar]
- Bansi R, Geeta C. Development and Validation of Stability Indicating RP-HPLC Method for Fenticonazole Nitrate in Capsule Dosage Form. Eur J Biomed Pharm Sci. 2018;5:701-7. [Google Scholar]
- Emannoaman M, Al-Ghobashyandhayamlotfy. Investigation of the Profile and Kinetics of Degradation of Fenticonazole Nitrate using Stability-indicating HPLC Assay in Presence of Methyl and Propyl Parabens. . Appl Preformul Stud TACL. 2016;6:850-62. [Google Scholar]
- Speed W, Jeffrey M, Roge S, Trevor E. The Development and Validation of a High-Performance Liquid Chromatography (HPLC)/Tandem Mass Spectrometry Assay for Fenticonazole in Human Plasma and Comparison with an HPLC-UV Method. Rapid Commun Mass Spectrometry. 1995;9(14):1452-6. [Google Scholar]
- Maoa W, Wang Y, Hua W, Jiao F, Fanc H, Ding L. Determination of fenticonazole in human plasma by HPLC-MS/MS and its application to pharmacokinetic studies. Journal of Pharmaceutical Analysis. 2016. [Google Scholar]
- Feng Z, Zou Q, Tan X, Che W, Zhang Z. Determination of fenticonazole enantiomers by LC-ESI-MS/MS and its application topharmacokinetic studiesin female rats. Arzneimittelforschung. 2011. [Google Scholar]
- Abstract
- Introduction
- Experimental
- Preliminary solubility studies and selection of solvent
- Preparation of stock standard solution
- Determination of λ max and calibration curve
- Analysis of Capsule formulation
- Accuracy/Recovery Studies
- Results and Discussion
- Conclusion
- Source of Funding
- Conflict of Interest
- References
How to Cite This Article
Vancouver
Gorle A, Jain P, Nerkar P, Haswani N, Chordiya S, Kommakayam SH. Quantitative estimation of fenticonazole nitrate by zero-order derivative area under curve spectrophotometric methods in bulk and in-capsule dosage form [Internet]. Int J Pharm Chem Anal. 2024 [cited 2025 Oct 21];11(2):175-179. Available from: https://doi.org/10.18231/j.ijpca.2024.025
APA
Gorle, A., Jain, P., Nerkar, P., Haswani, N., Chordiya, S., Kommakayam, S. H. (2024). Quantitative estimation of fenticonazole nitrate by zero-order derivative area under curve spectrophotometric methods in bulk and in-capsule dosage form. Int J Pharm Chem Anal, 11(2), 175-179. https://doi.org/10.18231/j.ijpca.2024.025
MLA
Gorle, Ashish, Jain, Pritam, Nerkar, Pankaj, Haswani, Nitin, Chordiya, Saurabh, Kommakayam, Saipudeen H.. "Quantitative estimation of fenticonazole nitrate by zero-order derivative area under curve spectrophotometric methods in bulk and in-capsule dosage form." Int J Pharm Chem Anal, vol. 11, no. 2, 2024, pp. 175-179. https://doi.org/10.18231/j.ijpca.2024.025
Chicago
Gorle, A., Jain, P., Nerkar, P., Haswani, N., Chordiya, S., Kommakayam, S. H.. "Quantitative estimation of fenticonazole nitrate by zero-order derivative area under curve spectrophotometric methods in bulk and in-capsule dosage form." Int J Pharm Chem Anal 11, no. 2 (2024): 175-179. https://doi.org/10.18231/j.ijpca.2024.025
