- Visibility 102 Views
- Downloads 26 Downloads
- Permissions
- DOI 10.18231/j.ijpca.2023.020
-
CrossMark
- Citation
Dietary phytochemicals and cancer chemoprevention
- Author Details:
-
Amar Arora
-
Neha Chauhan
-
Abid Ali Sheikh *
Abstract
The goal of cancer chemotherapy is to prevent or slow the growth of tumors by using various Biological or natural agents. Epidemiological and pre-clinical data indicate that phytochemicals can influence cell proliferation and cell cycle regulation, are typically involved in multiple signaling pathways that are frequently disrupted during tumour initiation, proliferation, and propagation, strengthen the host immune system, and make cancer cells more susceptible to cell-damaging agents. Only a small subset of these medicines have undergone clinical testing, and the results of those trials have been inconsistent, despite favorable outcomes from Experimental investigation. Recognizing the impact of these dietary changes may inspire easy and affordable solutions to improve health globally as the global rate of cancer continues to rise. In this article, we provide a summary of the information on a few phytochemicals with a particular focus on the clinical data demonstrating these compounds' effectiveness in populations at significant risk.
Cancer as Enigma
Despite the progress in treatment options and improvements in survival rate in certain types of tumors, cancer continues to be one of the significant causes of mortality worldwide. Globally, in 2021 there are an estimated 20 million new cases of cancer and 10 million cancer-related deaths. Over the next two decades, the cancer burden is expected to increase by approximately 60%, further increasing the strain on healthcare providers, individuals, and communities. The global burden is expected to rise to approximately 30 million additional cases of cancer by 2040, with the highest increase occurring in lower- and middle-income nations (as per WHO 2020).[1]
In India, the anticipated cancer burden for 2021 was 26.7 million, with an expected rise to 29.8 million by 2025. Over 40 percent of the total cancer burden was contributed by the seven leading cancer sites: the lung (10.6%), breast (10.5%), esophagus (5.8%), mouth (5.7%), stomach (5.2%), liver (4.6%), and cervix uteri (4.3%).[2]
Given these staggering demographics, as well as the associated suffering, pain, and economic stress, medical professionals and scientists are constantly striving for enhanced therapeutic options, optimized palliative care, as well as effective prevention measures. Therefore, the significance of diet in cancer has received considerable attention. This is particularly compelling in light of epidemiological analyses showing that consistent intake of phytochemicals from dietary sources such as vegetables, herbs, fruits, spices, and teas is linked to a lower risk of chronic illnesses such as cancer, heart diseases, and autoimmune conditions.[2], [3]
Cancer is the general name for over 100 medical conditions that involve uncontrolled and dangerous cell growth. It is a neoplastic condition resulting from genetic changes that control cell proliferation, maturation, metastatic behavior, and senescence. These errors may arise from certain causes, including mutations, chemical carcinogens, ionizing radiation, infection, hormonal imbalances, immune system dysfunction, heredity, environmental exposures, diet, and exercise. Other cancer-promoting genetic abnormalities may randomly occur through errors in DNA replication or are inherited and are thus present in all cells from birth. The heritability of cancer is usually affected by complex interactions between carcinogens and the host genome[4].Genetic abnormalities found in cancer typically affect two general classes of genes. Cancer-promoting proto-oncogenes and tumor suppressor genes. Most cancers can be treated and some cured, depending on the specific type, location, and stage,[5]
Detonations of scientific evidence seen in the past two decades documented that lifestyle is one of the principal causes in the pathophysiology of chronic diseases, including cancer.[6] The excess generation of ROS causes the implication in pathology and progression of carcinogenic transformation. The formation of byproducts from lipid peroxidation leads to loss of cellular integrity and, subsequently, cell pathology.[7] Therefore, establishing anticancer strategies by using methodical and scientific investigations from an enormous pool of biological & natural products to meet the improvement of therapeutic activity and sensitivity of anticancer agents.[8]
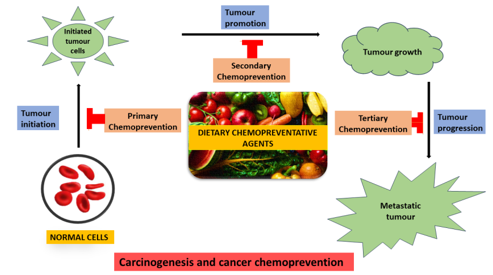
Exploring phytochemicals derived from natural sources may be feasible for cancer chemoprevention. They exhibit antioxidant and prooxidant properties that modulate cell proliferation and apoptotic pathways.[9] Chemoprevention is one of the growing areas of anticancer research with the focus on various interventions such as nutritional factors, biological and pharmacological. There are three different approaches involved in cancer chemoprevention: primary, secondary, and tertiary chemoprevention.
Hallmarks of Cancer
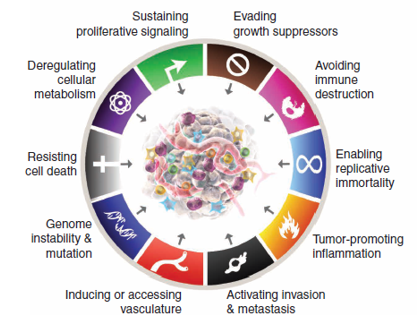
The Hallmarks of Cancer have been proposed to be a set of functional characteristics developed by human cells as they progress from normal to neoplastic development stages, particularly the capabilities critical for the formation of malignant tumors.
The hallmarks of cancer comprise of eight biological capabilities acquired during the multistep development of human cancers. They include sustaining proliferative signaling, evading growth suppressors, resisting cell death, enabling replicative immortality, inducing angiogenesis, and activating invasion and metastasis,reprogramming cellular metabolism, and avoiding immune destruction. In the most recent elaboration of this concept,[10] deregulating cellular metabolism and avoiding immune destruction were segregated as “emerging hallmarks,” but now, eleven years later, it is evident that they, much like the original six, can be considered core hallmarks of cancer, and are included as such in the current depiction in figure 1.
Cancer results from a multistage, multi-mechanism carcinogenesis process that involves mutagenic cell death and epigenetic mechanisms during three distinguishable but closely allied stages: initiation, promotion, and progression. Since reducing the initiation phase to a zero level is impossible, the most effective intervention would be at the promotion phase to eliminate premalignant cells before they become malignant.
Approaches and Procedures for Cancer Treatmet
Medical research on cancer has begun much like research on any disease. In organized studies of new treatments for cancer, the preclinical development of drugs, devices, and techniques begins in laboratories, either with isolated cells or in small animals, most commonly rats or mice. In other cases, the proposed treatment for cancer is already in use for other medical conditions, and more is known about its safety and potential efficacy. The presence of cancer can be suspected on the basis of the symptoms or radiological findings. However, definitive diagnosis of cancer requires microscopic examination of a biopsy specimen. Most cancers are treated accordingly. Prognosis is influenced by the type of cancer and extent of disease. Although cancer can affect people of all ages, the risk typically increases with age.
Conventional therapeutic approaches such as surgery, chemotherapy, and radiotherapy have been used in the past, although significant advances in recent years have included stem cell therapy, targeted therapy, ablation therapy, nanoparticles, natural antioxidants, radionics, chemodynamic therapy, sonodynamic therapy, and ferroptosis-based therapy. Stem cell therapy has proven promising in regenerating and repairing diseased or damaged tissues by targeting both primary and metastatic cancer foci, and nanoparticles have opened up new diagnostic and therapeutic possibilities. [11] The twin goals of this research are to determine whether the treatment actually works (efficacy) and whether it is sufficiently safe. Regulatory processes attempt to balance the potential benefits with the potential harms so that people who are given the treatment are more likely to benefit from it than to be harmed by it.
Chemotherapy and radiation, which primarily target DNA, are widely employed to eliminate tumour cells, however these therapies also increase DNA damage in (surrounding) healthy tissue, causing toxicities and accelerated ageing. [12] It is also clear that most chemotherapeutic targets damage DNA, which impairs DNA replication and synthesis. These parameters, along with the extent of drug exposure and the type of DNA or structural damage caused by chemotherapy, all have an impact on the degree of cancer therapy-induced toxicity. To improve the quality of life of patients with cancer and survivors, treatment-induced side effects must be further reduced.[12]
The chemoprevention of cancer promotes the use of natural and artificial mediators to disrupt carcinogenesis by interrupting or defeating specific molecular signaling systems.[13] There is growing evidence that naturally occurring phytochemicals help to prevent carcinogen exposure and carcinogenesis .Dietary phytochemicals are thought to have anti-cancer capabilities with little or no adverse effects,p [14] and they may also boost the effectiveness of chemotherapy or radiation, making them an important method for targeting Cancer Cells. [15]
Chemoprevention of Cancer
The field of cancer chemoprevention began in 1966 when Lee Wattenberg demonstrated that compounds associated with fruits and vegetables (indoles and isothiocyanates) could prevent cancer development in an animal model. Carcinogenesis progresses through multiple molecular mechanisms and signaling molecules. Chemoprevention of cancer promotes the administration of natural and synthetic mediators that inhibit carcinogenesis by inhibiting specific molecular signaling processes.[16]
In 1985, Wattenberg categorized chemopreventive agents into inhibiting agents for carcinogen formation, blocking agents, and suppressing agents. The first category prevents carcinogen synthesis from precursors, while the second category prevents carcinogen-induced mutations by limiting particular metabolic carcinogen activation pathways and aiding detoxification by trapping particular reactive oxygen species (ROS). The third type slows tumor growth by inhibiting cell proliferation and differentiation, leading to apoptosis, autophagy, and necrosis, among other processes. Most plant-based bioactive compounds inhibit cancer progression via a few of these routes. Chemoprevention is a pharmaceutical intervention used to prevent or reverse carcinogenesis. As illustrated in Fig. 2, chemoprevention affects and inhibits carcinogenesis at each stage. Chemopreventive substances have also been shown to boost the efficacy of standard chemotherapeutic medications. The primary objective of cancer chemoprevention is to minimize the prevalence of early stage cancer and prevent the secondary development of tumors.[17]
Over the years, many natural substances have been extensively studied for their potential utility in cancer prevention. The increasing volume of in vitro and in vivo data on the cancer chemopreventive and chemotherapeutic results of plant-derived compounds prompted scientists to conduct clinical trials concentrating on the pharmacokinetics, efficiency, and safety of the phytocompounds.
The concept of delaying or preventing this transformation remains a viable and attainable goal for the future.[18] Natural products consist of a wide variety of biologically active phytochemicals including phenolics, flavonoids, carotenoids and alkaloids which have been shown to suppress early and late stages of carcinogenesis. The American National Cancer Institute has identified about 35 plant-based foods containing 1,000 different phytochemicals that possess cancer-preventive properties. The most exciting findings have been achieved with antioxidant and their precursors, which are found in dark, leafy green vegetables and coloured fruits.[19]
Chemoprevention by edible phytochemicals is now considered to be an inexpensive, readily applicable, acceptable and accessible approach to cancer control and management. A wide range of different classes of bioactive molecules have been identified such as organosulphur compounds from garlic, polyphenols in green tea and curcumin in turmeric, resveratrol from grapes, quercetin from onions and apples, pruretin from plums and cherries described in table).[20] Natural products support the treatment of all phases of cancer or interact with several targets simultaneously and often synergistically. These bioactive polyphenols can influence signal transduction factors, inhibit COX-2, promote cell cycle arrest, increase apoptosis and double multi-drug resistant pumps, curcumin from turmeric has been found to influence sixty such molecular targets in cancer treatment process.[21] The National Cancer Institute (NCI-USA) has more than 400 potential agents under investigation and is sponsoring more than 65 Phase I, Phase II and Phase III chemoprevention trials. These involve various substances or their mixtures, many of which are food borne phytochemicals. The much lower risk of colon, prostate and breast cancers in Asians, who generally consume more vegetables, fruits and tea than populations in the West raise the importance of flavonoid components mediated protective effects by acting as natural chemopreventive and chemotherapeutic agents.[22] Moreover, when used with chemotherapy agents such as 5-fluorouracil and doxorubicin, antioxidants enhance the cytotoxicity of chemotherapy agents and cause complete remissions, where only partial remission is possible with chemotherapy agents only.[23] Due to repeated epidemiological studies providing data about correlations between fruit consumption and reduced risk of chronic diseases, fruits have gained an important place in human nutrition.[24]
Natural bioactive Agents Used in Cancer Chemoprevention
|
S. No. |
Name of Phytocompound |
Major targets |
References |
|
1 |
Curcumin |
Cyclooxygenase-2 (COX-2), nuclear factor kappa B (NF-kB), tumor necrosis factor-alpha (TNF-a), and cyclin D1 |
|
|
2 |
Resveratrol |
12-O-tetradecanoylphorbol-13-acetate (TPA) in human leukemia HL-60 cells, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)–induced expression of cytochrome P450 1A1 (CYP1A1) and 1B1 (CYP1B1) andNAD(P)H: quinone oxidoreductase-1 (NQO1) in human leukemia K562 cells |
|
|
3 |
Apigenin |
NAG-1, p21, p53, ICAM-1, cyclin D1, c-myc, TNF and IL-1 |
|
|
4 |
Epigallocatechin gallate |
MMP-2, MMP-9, IGF-1R, DNMT1, DNMT2B, HSP90, Vimentin, Bcl-2, and TRAF-6 |
|
|
5 |
Genistein |
Tumor necrosis factor-α (TNF-α), Polo-like kinase 1 (PLK1), estrogen receptors (ER), protein tyrosine kinases (PTK) and mammalian DNA topoisomerase II |
Chemopreventive mechanisms & major signaling pathways
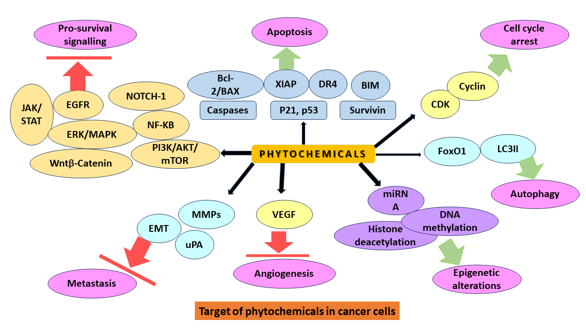
Natural chemopreventive drugs, particularly those derived from plants, have encouraged researchers to conduct numerous experiments to examine their underlying causes of action in cancer prevention.[31] Plant-based compounds or phytochemicals disrupt cellular mechanisms, such as inhibiting the expression of genes that suppress cancer progression, antioxidant enzyme action, cell cycle arrest, and reducing oxidative stress, autophagy, necrosis, and apoptosis, leading to oncogene activation, modulation of signaling pathways, and inhibition of angiogenesis and metastasis. Targeted compounds trigger cancer chemoprevention in all of these and many other pathways.[32]
Kinases, receptors, caspases, tumor suppressor proteins, transcriptional factors, miRNAs, and cyclins are some of these target molecules. Oncogenes are activated as a result of internal or external stimuli, resulting in carcinogenesis. In contrast, cells activate self-defense mechanisms such as autophagy, cell cycle arrest, and apoptosis by modifying cell signalling pathways. Phytochemicals can expedite these defensive mechanisms in the cellular matrix, cytoplasm, and nucleus by regulating numerous molecules that determine cell fate.[33]
Chemopreventive mechanisms & major signaling pathways
MAPK Pathways
Phytochemicals influence cellular development and survival by targeting extracellular signal-regulated kinase (ERK) and mitogen-activated protein kinase (MAPK) pathways. These plant-derived natural chemicals can suppress cancer growth via various pathways. [34] Ursolic acid, kaempferol, resveratrol, gingerol, sulforaphane, genistein, and isothiocyanates are among the key plant chemicals that have been demonstrated to initiate apoptosis via the MAPK and ERK pathways.[35]
Akt signaling pathways
The Akt/PI3 signaling pathway is critical for cancer control and progression. Epidermal growth factor (EGF) regulates a number of molecular mechanisms, including NF-κB activation and Akt phosphorylation, which leads to resistance to uncontrolled cell proliferation and apoptosis, downstream regulation of caspases, Bcl-2, glycogen synthase kinase 3-beta (GSK3), and mammalian target of rapamycin(mTOR). Alkaloids and phenolics significantly modulate the expression of these factors. Resveratrol, luteolin, apigenin, flavone, sulforaphane, and curcumin have been found to exhibit anticancer activity via cell cycle arrest and apoptosis, suppression of Akt/PI3K signaling, proapoptosis, FOXO3a (forkhead box O3) activation, antiproliferation, and anti-invasion. [36]
JAK/STAT signaling pathways
When Janus kinases (JAKs) are activated, they phosphorylate signal transducers and activators of transcription (STATs) and translocate to the nucleus where they control the transcription of p53, Bcl-2, cyclin D, and interlukin-6 (IL-6) involved in cell death, proliferation, and apoptosis. [37] Plant-derived compounds dramatically promote cell death in several cancer types by blocking JAK/STAT signalling and activating apoptotic cascades. [38]
Wnt/β-Catenin signaling pathways
Common malignancies, such as breast, lung, colon, blood, ovarian, skin, and brain tumours, are usually related with aberrant Wnt/β-catenin signaling.[39]
This pathway is activated by the binding of Wnt protein to frizzled family transmembrane receptors and the accumulation of β-catenin in the nucleus, which leads to the activation of transcriptional factors that regulate cell proliferation, survival, and migration.[40] Phytochemicals like Curcumin, resveratrol, and epigallocatechin-3-gallate (EGCG) inhibit the β-catenin translocation and accumulation in the nucleus through activating glycogen synthase kinase 3 (GSK3).[41]
P53 tumor suppressor
p53, in other signaling pathways, is responsible for the anticancer activity in cells. This tumor suppressor protein promotes the activation of the apoptotic cascade.[42] Various phytochemicals, such as resveratrol, Quercetin, EGCG, and piceatannol, have been shown to elevate p53, decrease Akt, and promote cellular apoptosis and cell cycle rest.[42] Another substantial contribution is the cross-linking of the p53, MAPK, and JNK pathways.[43] Curcumin and ursolic acid have been shown to stimulate autophagy, in which cells shut down aberrant proliferation and progression. Autophagy is promoted in cancer cells by inhibiting the Akt/mTOR pathway, which regulates inflammation, angiogenesis, invasion, and metastasis in tissues.[44], [45]
Conclusion
Dietary elements addressed in present review may be efficient cancer chemopreventive agents, and consumption of food containing specific bioactive chemicals has been demonstrated to have both protective and therapeutic effects on numerous types of cancer. Plant-based polyphenolic compounds have immunomodulatory properties that identify and destroy cancer cells through anti-angiogenic measures. Chemopreventive medications improve chemo and radiation efficacy through a variety of signal transduction mechanisms. Chemopreventive medications improve chemo-and radiation efficacy through various signal transduction mechanisms. As oxidative stress is involved in the etiology of many malignancies, the antioxidant action of dietary phenolic compounds might offer a promising cancer prevention approach.
Source of Funding
None.
Conflict of Interest
None.
References
- . World Cancer Day 2023: Close the care gap. America: PAHO. Available. . [Google Scholar]
- Kulothungan V, Kumar K, Leburu S, Ramamoorthy T, Stephen S, Basavarajappa D. Burden of cancers in India-estimates of cancer crude incidence, YLLs, YLDs and DALYs for 2021 and 2025 based on National Cancer Registry Program. BMC Cancer. 2022;22(1). [Google Scholar]
- Chikara S, Nagaprashantha L, Singhal J, Horne D, Awasthi S, Singhal S. Oxidative stress and dietary phytochemicals: role in cancer chemoprevention and treatment. Cancer Lett. 2018;413:122-56. [Google Scholar]
- Anand P, Kunnumakkara A, Harikumar K, Ahn K, Badmaev V, Aggarwal B. Modification of cysteine residue in p65 subunit of nuclear factor-κB (NF-κB) by Picroliv suppresses NF-κB-regulated gene products and potentiates apoptosis. Cancer Res. 2008;68(21):8861-70. [Google Scholar]
- Macdiarmid J, Amaro-Mugridge N, Madrid-Weiss J, Sedliarou I, Wetzel S, Kochar K. Sequential treatment of drug-resistant tumors with targeted minicells containing siRNA or a cytotoxic drug. Nat Biotechnol. 2009;27(7):643-51. [Google Scholar]
- Sharifi-Rad M, Kumar A, Zucca N, Varoni P, Dini E, Panzarini L. Lifestyle, oxidative stress, and antioxidants: back and forth in the pathophysiology of chronic diseases. Front Physiol. 2020;11. [Google Scholar]
- Juan C, Lastra PDL, Plou J, Pérez-Lebeña F. The chemistry of reactive oxygen species (ROS) revisited: outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int J Mol Sci. 2021;22(9). [Google Scholar]
- Rayan A, Raiyn J, Falah M. Nature is the best source of anticancer drugs: Indexing natural products for their anticancer bioactivity. PloS one. 2017;12(11). [Google Scholar]
- Choudhari A, Mandave P, Deshpande M, Ranjekar P, Prakash O. Phytochemicals in cancer treatment: From preclinical studies to clinical practice. Front Pharmacol. 2020;10. [Google Scholar] [Crossref]
- Hanahan D, Weinberg R. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646-74. [Google Scholar]
- Debela D, Muzazu S, Heraro K, Ndalama M, Mesele B, Haile D. New approaches and procedures for cancer treatment: Current perspectives. SAGE Open Med. 2021;9. [Google Scholar] [Crossref]
- Boogaard WVD, Komninos D, Vermeij W. Chemotherapy Side-Effects: Not All DNA Damage Is Equal. Cancers. 2022;14(3). [Google Scholar]
- Abotaleb M, Samuel S, Varghese E, Varghese S, Kubatka P, Liskova A. Flavonoids in Cancer and Apoptosis.. Cancers. 2019;11(1). [Google Scholar] [Crossref]
- Varghese E, Samuel S, Varghese S, Cheema S, Mamtani R, Büsselberg D. Triptolide Decreases Cell Proliferation and Induces Cell Death in Triple Negative MDA-MB-231. Breast Cancer Cells. Biomol. 2018;8(4). [Google Scholar] [Crossref]
- Lee C, Chen C. Natural product-based therapeutics for the treatment of cancer stem cells: A patent review. Expert Opin Ther Pat. 2010;25(6). [Google Scholar] [Crossref]
- Macdiarmid J, Amaro-Mugridge N, Madrid-Weiss J, Sedliarou I, Wetzel S, Kochar K. Sequential treatment of drug-resistant tumors with targeted minicells containing siRNA or a cytotoxic drug. Nat Biotechnol. 2009;27(7):643-51. [Google Scholar]
- Sharma G, Tyagi A, Singh R, Chan D, Agarwal R. Synergistic Anti-Cancer Effects of Grape Seed Extract and Conventional Cytotoxic Agent Doxorubicin against Human Breast Carcinoma Cells. Breast Cancer Res Treat. 2004;85(1):1-12. [Google Scholar]
- Brenner D, Gescher A. Cancer chemoprevention: lessons learned and future directions. Br J Cancer. 2005;93(7):735-44. [Google Scholar]
- Karikas G. Anticancer and chemopreventing natural products: some biochemical and therapeutic aspects. J BUON. 2010;15(4):627-65. [Google Scholar]
- Gray W, Hopkins L, Yadav S, Davis T, Wholey M, Atkinson R. Protected carotid stenting in high-surgical-risk patients: the ARCHeR results. J Vasc Surg. 2006;44(2):258-68. [Google Scholar]
- Ohori H, Yamakoshi H, Tomizawa M, Shibuya M, Kakudo Y, Takahashi A. Synthesis and biological analysis of new curcumin analogues bearing an enhanced potential for the medicinal treatment of cancer. Mol Cancer Ther. 2006;5(10):2563-71. [Google Scholar]
- Middleton E, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000;52(4):673-751. [Google Scholar]
- Herrmann K. Flavonols and flavones in food plants: a review. Int. J. Food Sci. 1976;11(5):433-481. [Google Scholar]
- He L, He X, Lim LP, Stanchina D, E, Liang XZ, et al. A microRNA component of the p53 tumour suppressor network. Nature. 2007;447(7148):1130-1134. [Google Scholar]
- Negri A, Naponelli V, Rizzi F, Bettuzzi S. Molecular targets of epigallocatechin-Gallate (EGCG): A special focus on signal transduction and cancer. Nutr. 2018;10(2). [Google Scholar] [Crossref]
- Ko JH, Sethi G, Um JY, Shanmugam MK, Arfuso F, Kumar AP. The role of resveratrol in cancer therapy. Int. J. Mol. Sci. 2017;18(12):2589-2589. [Google Scholar]
- George BP, Chandran R, Abrahamse H. Role of phytochemicals in cancer chemoprevention. Insights. Antioxidants. 2021;10(9):1455-1455. [Google Scholar]
- Zhong Y, Krisanapun C, Lee S, Nualsanit T, Sams C, Peungvicha P. Molecular targets of apigenin in colorectal cancer cells: involvement of p21, NAG-1 and p53. Eur J Cancer. 2010;46(18):3365-74. [Google Scholar]
- Chae H, Xu R, Won J, Chin Y, Yim H. Molecular targets of genistein and its related flavonoids to exert anticancer effects. Int J Mol Sci. 2019;20(10). [Google Scholar]
- Russo M, Russo G, Daglia M, Kasi P, Ravi S, Nabavi S. Understanding genistein in cancer: The “good” and the “bad” effects: A review. Food Chem. 2016;196:589-600. [Google Scholar]
- Patterson S, Maresso C, Hawk K. Cancer chemoprevention: successes and failures. Clin Chem. 2013;59(1):94-101. [Google Scholar]
- Hu R, Kong A. Activation of MAP kinases, apoptosis and nutrigenomics of gene expression elicited by dietary cancer-prevention compounds. Nutr. 2004;20(1):83-8. [Google Scholar]
- Michl C, Vivarelli F, Weigl J, Nicola D, Canistro G, Paolini D. The chemopreventive phytochemical moringin isolated from Moringa oleifera seeds inhibits JAK/STAT signaling. PloS one. 2016;11(6):83-8. [Google Scholar]
- Cohen S, Arnold L. Chemical carcinogenesis. Toxicol Sci. 2011;120:76-92. [Google Scholar]
- Adachi S, Shimizu M, Shirakami Y, Yamauchi J, Natsume H, Matsushima-Nishiwaki R. Epigallocatechin gallate downregulates EGF receptor via phosphorylation at Ser1046/1047 by p38 MAPK in colon cancer cells. Carcinog. 2009;30(9):1544-52. [Google Scholar]
- Prasad R, Vaid M, Katiyar S. Grape proanthocyanidin inhibit pancreatic cancer cell growth in vitro and in vivo through induction of apoptosis and by targeting the PI3K/Akt pathway. PLoS One. 2012;7(8). [Google Scholar] [Crossref]
- Yu H, Lee H, Herrmann A, Buettner R, Jove R. Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat Rev Cancer. 2014;14(11):736-82. [Google Scholar]
- Kim D, Park K, Chae I, Kundu J, Kim E, Kundu J. Carnosic acid inhibits STAT3 signaling and induces apoptosis through generation of ROS in human colon cancer HCT116 cells. Mol Carcinog. 2016;55(6):1096-110. [Google Scholar]
- Wang D, Wise M, Li F, Dey M. Phytochemicals attenuating aberrant activation of β-catenin in cancer cells. PloS one. 2012;7(12). [Google Scholar] [Crossref]
- Taipale J, Beachy P. The Hedgehog and Wnt signalling pathways in cancer. Nature. 2001;411(6835):349-54. [Google Scholar]
- Wang D, Wise M, Li F, Dey M. Phytochemicals attenuating aberrant activation of β-catenin in cancer cells. PloS one. 2012;7(12). [Google Scholar]
- Toshiyuki M, Reed J. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995;80(2):293-302. [Google Scholar]
- Herrera I, Martín M, Bravo L, Goya L, Ramos S. Epicatechin gallate induces cell death via p53 activation and stimulation of p38 and JNK in human colon cancer SW480 cells. Nutr Cancer. 2013;65(5):718-46. [Google Scholar]
- Wung B, Hsu M, Wu C, Hsieh C. Resveratrol suppresses IL-6-induced ICAM-1 gene expression in endothelial cells: effects on the inhibition of STAT3 phosphorylation. Life sci. 2005;78(4):389-97. [Google Scholar]
- Siddiqui A, Jahan S, Singh R, Saxena J, Ashraf S, Khan A. Plants in anticancer drug discovery: from molecular mechanism to chemoprevention. Biomed Res. Int. 2022. [Google Scholar] [Crossref]
- Abstract
- Cancer as Enigma
- Hallmarks of Cancer
- Approaches and Procedures for Cancer Treatmet
- Chemoprevention of Cancer
- Natural bioactive Agents Used in Cancer Chemoprevention
- Chemopreventive mechanisms & major signaling pathways
- Chemopreventive mechanisms & major signaling pathways
- Akt signaling pathways
- JAK/STAT signaling pathways
- Wnt/β-Catenin signaling pathways
- P53 tumor suppressor
- Conclusion
- Source of Funding
- Conflict of Interest
- References
How to Cite This Article
Vancouver
Arora A, Chauhan N, Sheikh AA. Dietary phytochemicals and cancer chemoprevention [Internet]. Int J Pharm Chem Anal. 2023 [cited 2025 Sep 23];10(2):110-115. Available from: https://doi.org/10.18231/j.ijpca.2023.020
APA
Arora, A., Chauhan, N., Sheikh, A. A. (2023). Dietary phytochemicals and cancer chemoprevention. Int J Pharm Chem Anal, 10(2), 110-115. https://doi.org/10.18231/j.ijpca.2023.020
MLA
Arora, Amar, Chauhan, Neha, Sheikh, Abid Ali. "Dietary phytochemicals and cancer chemoprevention." Int J Pharm Chem Anal, vol. 10, no. 2, 2023, pp. 110-115. https://doi.org/10.18231/j.ijpca.2023.020
Chicago
Arora, A., Chauhan, N., Sheikh, A. A.. "Dietary phytochemicals and cancer chemoprevention." Int J Pharm Chem Anal 10, no. 2 (2023): 110-115. https://doi.org/10.18231/j.ijpca.2023.020
