- Visibility 1.8k Views
- Downloads 233 Downloads
- Permissions
- DOI 10.18231/j.ijpca.2023.004
-
CrossMark
- Citation
Recent advances in biological applications of mannich bases — An overview
Abstract
Mannich bases are beta-amino ketone-carrying compounds that are the end products of the Mannich reaction. The Mannich reaction is essential in the synthesis of nitrogen-containing compounds. The Mannich reaction, a nucleophilic addition reaction that forms carbon-carbon bonds, is employed in the synthesis of numerous natural products, drugs and other compounds. There are several aminoalkyl chain bearing Mannich bases with high curative value, such as fluoxetine, atropine, ethacrynic acid, trihexyphenidyl, and many others. According to the literature, Mannich bases are highly reactive and have been demonstrated to have powerful anti-inflammatory, anticancer, antifilarial, antibacterial, antifungal, anticonvulsant, anthelmintic, antitubercular, analgesic, anti-HIV, antimalarial, antipsychotic, antiviral, antilipase activity and other properties. The primary source of, -α,β-unsaturated ketone, which can be produced by deaminating the hydrogen atom of the amine group, is responsible for the biological action of Mannich bases. However, a number of minor biological functions of Mannich bases have been reported, including their ability to control blood pressure or prevent platelet aggregation, their impact on parasites and ulcers, and their use as medications for mental health issues. Since there is a greater need for anticonvulsant pharmaceuticals, these anticonvulsant medications containing N-mannich base groups will exhibit an increase in lipophilic nature and hence have improved blood-brain barrier penetration. The overview concludes with a brief explanation of the potential of Mannich bases as inhibitors of various enzymes or ligands for various receptors.
Introduction
The most basic method of introducing C-C bond formation in organic synthesis is the Mannich reaction. Aldehyde, ketone and a secondary amine make up the three-component complex known as mannich base. The mannich base's antimicrobial, antimalarial, anti-HIV, anti-tubercular, anti-cancer, anti-inflammatory and anti-oxidant activities make them interesting pharmacologically. The complexity of aldehydes, ketones and amines had a major impact on the individual optimization of time, temperature and percentage yield of synthesized compounds. In the area of medicinal chemistry, the Mannich base has found several practical applications and it may be responsible for increasing physicochemical qualities. Mannich bases and their derivatives are being developed with the aim of improving their physicochemical and druglike characteristics, which may have greater influence over pharmacological applications.[1], [2], [3]
Many bioactivities, such as antineoplastic, diuretic, antipsychotic, anticonvulsant, centrally acting muscle relaxant, antibacterial and antiviral ones, are present in the Mannich base of heterocyclic compounds. Carbazole mannich bases serve as a helpful model for the identification and creation of prospective anti-cancer drugs. Lewis’s acids and bases, Bronsted acids, rare metal salts and organocatalysts can be used to catalyze the traditional Mannich reactions of aldehydes, ketones and amines. However, these catalysts come with a number of drawbacks that restrict their use, including difficult reaction product separation, prolonged reaction durations and harsh reaction conditions. Because of the significant pharmacophores with promising activity, Mannich bases generated from acetophenone are particularly interesting and have shown significant potential in medicinal chemistry and drug development. They frequently serve as starting points for the production of novel pharmacophores or medicines with therapeutic potential. Recently, several mannich bases with strong clinical support have been reported. In medicine, mannich base and its derivatives are regarded as the best pharmacophore.[4], [5], [6]
The phenolic mannich base, which has the structural components of a phenolic hydroxyl group and an aminoalkyl moiety, exhibits a wide range of biological activities, including anti-oxidant, anti-inflammatory, biometal chelating, anticholinesterase, and monoamino oxidase inhibitory properties. Apoptosis, cell cycle arrest, depolarization of the mitochondrial membrane and poorer topoisomerase-II inhibition were all a result of the mannich base's cytotoxicity. A potent technique in medicinal chemistry, the Mannich base reaction helps to create novel chemical entities or improves the physicochemical characteristics of drug candidates.[7], [8], [9]
Many herbicidally active compounds have been prepared using the Mannich base reaction. The Mannich reaction could also be used to manufacture several other neonicotinoid pesticides. A helpful strategy for creating enantioenriched aminocarbonyl compounds with favourable pharmacological characteristics is the asymmetric form of the catalytic Mannich process. The microbiological properties of an amino methylated Mannich base and their properties for complexing with divalent transition metals may be improved. Metal complexes are also employed as preventative drugs in the treatment of infectious diseases.[10], [11], [12]
It is claimed that mannich bases made from isatin derivatives have a variety of biological roles. They examined the in vitro inhibitory activity of the newly synthesized N-mannich base against a panel of gram-positive and gram-negative bacteria. The mannich base reaction has emerged as a key component in multidrug synthesis, enabling the quick and easy synthesis of a wide range of antibacterial, cytotoxin, and anticonvulsant substances. The associated heterocyclic intermediates also give rise to heterocyclic mannich bases, which also have a number of advantageous characteristics. But the majority of them engaged in empathetic pharmaceutical activities.[13], [14], [15], [16]
In this review, various biological applications of mannich bases are given based on the literature survey between the time period of 2012 to 2023. Only those kinds of literature indexed in Google Scholar, PubMed, Scopus, ScienceDirect, Embase, Web of Science and ResearchGate were collected by employing mannich base, biological applications, activities, antimicrobial, anticancer, therapeutic potential, efficacy as keywords, individually and in combination. Thus, it will be useful to design and develop novel mannich base and derivatives with potential applications.
Antimicrobial Activity of Mannich bases
Antimicrobials are viewed as one of the most successful forms of therapy in medicine, though their efficacy is adversely affected by antibiotic-resistant pathogens. Generally, mannich bases were synthesized by the reaction of an aldehyde, ketone and an amine. Thiosemicarbazide was condensed with the synthesized mannich bases to create thiosemicarbazide mannich bases. The compounds were examined using the BHI (brain heart infusion) broth dilution method for antifungal activity against Candida albicans and Aspergillus niger. Both C. albicans and A. niger have demonstrated comparable efficacy for alkyl components. In comparison to A. niger, aliphatic carbonyl compounds demonstrated good antifungal activity against C. albicans. Using fluconazole as the standard drug, the compound (1) (2E)-2[4-(morpholin-4-yl) butan-2-ylidene]hydrazine-1-carbothioamide demonstrated the highest activity against C. albicans and A. niger.[2]
Some medications that contain a significant amount of imidazole nucleus include fungicides, angiotensin II receptor antagonists and oral anti-inflammatory medicines. The synthesis of many 3-substituted aminomethyl-5-(2-methyl-4-nitro-1-imidazomethyl)-1,3,4-oxadiazole-2-thiones with different substituent groups was described using a general and practical approach. Likewise, the antimicrobial activity of the newly synthesised Mannich bases was shown. When compared to the reference drug fluconazole, 3-substituted aminomethyl-5-(2-methyl-4-nitro-1-imidazomethyl)-1,3,4-oxadiazole-2-thiones (2,3,4) had greater inhibitory activity against C. albicans, Trichophytonmentagrophytes and S. aureus. Compared to fluconazole, the compounds with ethyl, fluoro, and bromo groups at para position in phenyl substituent showed higher activity against T. mentagrophytes.[14]
In addition to the tri-component synthesis of novel chiral benzimidazole Mannich bases via reaction between benzimidazole, aqueous 30% formaldehyde and an amine, the biological evaluation and DFT investigations of the new compounds were also presented. By comparing the minimal inhibitory concentration values of benzimidazole compounds to a reference drug, erythromycin, the antibacterial activity of these compounds was assessed against bacterial strains, including Gram-positive cocci, Staphylococcus aureus and Gram-negative bacilli, Pseudomonas aeruginosa and Escherichia coli. Inhibiting the growth of gram-negative microorganisms, the compound (5) (S)-1-(1-((Diphenylamino)-1H-benzo (d) imidazole-2-ylethanol proved effective against the tested strains. The antibacterial activity of the compound (6) (S)-1-(1 ((4 nitrophenylamino) methyl)-1H-benzo[d] imidazole 2 yl) ethanol has been demonstrated better activity against the Gram-negative bacilli strain P. aeruginosa.
Phenolic components such as eugenol have good antifungal activity against Candida spp. When tested against Candida spp., the majority of the eugenol derivatives demonstrated significant antifungal activity, demonstrating that eugenol can produce better molecules when its structural properties are changed. Among the synthesized compounds, 4-allyl-2-methoxy-6-(morpholin-4-ylmethyl) phenyl benzoate (7) and 4-{5-allyl-2- [(4-chlorobenzoyl)oxy]-3-methoxybenzyl}morpholin-4-ium chloride (8) were found to be the most effective antifungal compounds with low IC50 values.[17]
A number of novel Mannich base derivatives of flavones (9, 10) with a benzylamine moiety were created using the Mannich reaction. The antifungal activity results are not ideal, but it has a greater antifungal effect than flavonoids. Following that, four bacteria were used to test these compounds antibacterial activity, which demonstrates better activity against S. aureus and Salmonella gallinarum. When compared to novobiocin, tested compounds demonstrated broad spectrum antibacterial activity against two gram-positive bacteria, S. aureus and Listeria monocytogenes, as well as two gram-negative bacteria, E. coli and S. gallinarum. The selected compounds inhibit Topo II and Topo IV with better IC50.[18] A series of Mannich base derivatives Allyl-6-(4-alkylphenoxy)-3-((diethylamino) methyl)benzene-1,2-diol) and 4-alkll-6-(-alkllphenoxy)-3-((4-ethy)piperazine-1-yl)methyl)benzene-1,2-diol) (11,12) were prepared by the C-4-amino methylated modification of obovatol and all synthesized compounds were tested for in vitro antifungal activity against several phytopathogenic fungi using the spore germination method. The presence of diethylamino, pyrrolyl, 1-methyl-piperazinyl, or 1-ethyl-piperazinyl groups to the C-4 position of obovatol may increase the probability of providing potential antifungal compounds. compounds inhibited Botrytis cinerea spore germination more effectively than two positive controls, hymexazol and difenoconazole.[19]
Mannich base of triazole derivatives containing nitro substituted thiophene proved highly promising antimicrobial activity. It was carried out in Mueller-Hinton broth. The compounds (13) 1-((1-(morpholinomethyl)-4-(5-nitrothiophene-2-yl) methylene amino)-5-thioxo-4,5-dihydro-1H-1,2,4-triazol-3-yl) methyl)-4- ((5-nitrothiophene-2-yl) methyleneamino)-3-(thiophene-2-yl methyl)-1H-1,2,4-triazole-5(4H)-one was more effective against gram-negative bacteria than against gram-positive bacteria.[20]
A series of thieno[2,3-d]pyrimidine alkyne Mannich base hybrid (14) was designed, synthesised and tested for concentration-dependent antibacterial activity. Structure activity relationship (SAR) studies revealed that changing the substitutions on the C4 position of the thienopyrimidine ring and the terminal phenyl ring causes significant changes in biological activities. It was analyzed against four bacterial strains and was effective against E. coli.[21]
The anti-Leishmanial capability of many novel lawsone Mannich bases produced from salicylaldehydes or nitro furfural against Toxoplasma gondii and Trypanosoma brucei parasites was examined. The antiparasitic activity of Lawsone Mannich bases against T. brucei and Entamoeba histolytica has been particularly promising. Compound (15), 3‐[(Hexadecylamino)(2‐hydroxyphenyl)methyl]‐2‐hydroxy‐1,4‐ naphthoquinone was a promising drug candidate for toxoplasmosis treatment.[22] Using a molecular hybridization strategy, design, synthesis and testing of 6-fluoroquinazolinylpiperidinyl -modified 1,2,4-triazole-based Mannich base derivatives (16) as antibacterial agents against phytopathogenic bacteria and fungi. The results of the turbidimetric experiments against Xanthomonas oryzae pv. oryzae showed that certain drugs exhibited high in vitro antibacterial therapeutic activity.[23] Analogues of N(benzimidazol-1-ylmethyl)-4- chlorobenzamide were created using the Mannich process. The synthesized derivatives were evaluated for in vitro antimicrobial activity against gram-negative, gram-positive and fungal strains. The most potent compound among the produced derivatives was determined to be derivative (17) N-[2-(2-chloro-phenyl)-benzimidazol-1-ylmethy]-4-chlorobenzam, which has a 2-chlorophenyl moiety at position 2 on the benzimidazole scaffold.[24] The structure of the compound possessing antimicrobial activity is given in [Figure 1], [Figure 2].
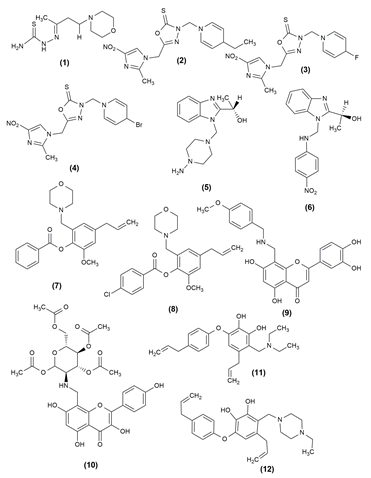
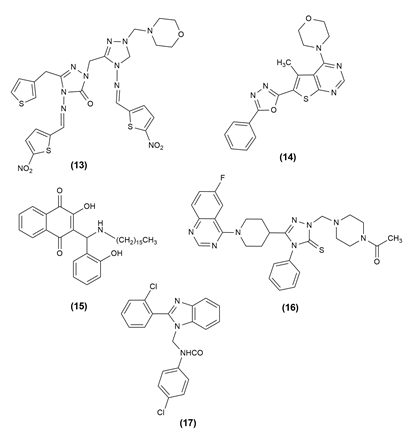
Anticancer Activity of Mannich bases
There are numerous life-threatening diseases prevalent worldwide, and cancer has gradually become a major threat to humans. The compounds were tested for antiproliferative activity against prostate cancer (PC3), Hep G2 (liver cancer), human colorectal cancer (HCT-116), human hepatocellular carcinoma (HePG-2), human epithelioid carcinoma (HeLa) and human breast cancer (MCF7) cell lines. In almost all of the cancer cell lines tested, the given compounds in [Table 1] inhibited cell proliferation effectively. To assess the anticancer properties of the novel compounds, in vitro cytotoxicity experiments using the lung cancer cell lines A549 and H1975 were performed.[25], [26] [Table 1] shows the various compounds possessing anticancer activity with the effective cell line and their structures are given in [Figure 3], [Figure 4], [Figure 5].
|
Compound No |
Compound Name |
Cancer Cell Line (Type) |
Ref. |
|
18 |
1-Cyclopropyl-6-fluoro-7-(4-((1-hydroxynaphthalen-2-yl)methyl) piperazin-1-yl)-4-oxo-1,4 dihydroquinoline-3-carboxylic acid |
HOP-92 (lung) |
|
|
19 |
3-((4-Bromophenyl)(pyrrolidin-1-yl)methyl)-9H-carbazol’4-ol |
Hela (cervical) MDA-MB-231 (breast) |
|
|
20 |
N-((E)-5-(3-Hydroxyphenyl)-2-((E)-3-(3-hydroxyphenyl)- acryloyl)-3-oxo-1-phenylpent-4-en-1-yl)-acrylamide |
A549 (lung) H1975 (lung) |
|
|
21 |
3-[(3-chlorophenylamino)methyl]-5-(3,4-dimethoxyphenyl)-1,3,4-oxadiazole-2(3H)-thione |
MCF-7 (breast) HeLa (cervical) |
|
|
22 |
5-(3,4-Dimethoxyphenyl)-3-{4-[(2-trifluorobenzyl)piperazin-1-yl]methyl}-1,3,4-oxadiazole-2(3H)- thione |
HeLa (cervical) MFC-7 (breast) |
|
|
23 |
(E)-3' [(Dimethylamino) methyl] pterostilbene |
HeLa (cervical) |
|
|
24 |
1-3-bis-[(3-hydroxy-naphthalen-2-yl)-phenyl-methyl]-urea] |
HEP-G2 (liver) |
|
|
25 |
12-(1-(2-chlorophenyl)piperazine-1-ylmethyl)-berber-rubine |
Caski (cervical) |
|
|
26 |
[(2Z)‑6‑(Acetyloxy)‑2‑(3,4‑dimethoxybenzylidene)‑3‑oxo‑2,3‑dihydro‑1‑benzofuran‑7‑yl]methyl acetate |
PC-3 (prostate) |
|
|
27 |
[(2Z)‑6‑(Acetyloxy)‑2‑(1,3‑benzodi‑ oxol‑5‑ylmethylene)‑3‑oxo‑2,3‑dihydro‑1‑benzofuran‑7‑yl] methyl acetate |
PC-3 (prostate) |
|
|
28 |
3,5,7-Trihydroxy-2-(4-methoxyphenyl) -6,8-bis [(dimethylamino) methyl]-4H-chromen -4-one |
HeLa (cervical) |
|
|
29 |
3,5,7-Trihydroxy-2-(4-methoxyphenyl) -6,8-bis [(dipropylamino) methyl]-4H-chromen-4-one |
HCC1954 (breast) SK-OV-3 (ovarian) |
|
|
30 |
5-(4-chlorophenyl) -3-(((4 hydroxyphenyl) amino)methyl)-1,3,4-oxadiazole2( 3H)-thione |
MCF-7 (breast) |
|
|
31 |
2-(2-chlorophenyl) -5-(2-fluorophenyl) -1,3,4-oxadiazole |
MCF-7 (breast) MDA-MB-231 (breast) |
|
|
32 |
2-(2-chlorophenyl) -5-(2 methoxyphenyl) -1,3,4-oxadiazole |
MCF-7 (breast) MDA-MB-231 (breast) |
|
|
33 |
(5β,7α,9β,10α)-7,14-Dihydroxykaur-17-(4-methyl) aniline 3,15-dione. |
A549 (lung) |
|
|
34 |
(5β,7α,9β,10α)-7,14-Dihydroxykaur-17-cyclohexyl-3,15- dione |
A549 (lung) |
|
|
35 |
4’,7-Dimethoxy-5-hydroxy-6-[(dimethylamino) methyl]- flavothione |
HeLa (cervical) HCC1954 (breast) SK-OV-3 (ovarian) |
|
|
36 |
4’,7-Dimethoxy-5-hydroxy-6-[(piperidin-1-yl)methyl]- flavothione |
HeLa (cervical) HCC1954 (breast) SK-OV-3 (ovarian) |
|
|
37 |
2-Phenyl-3-(pyrrolidin-1-ylmethyl)imidazo[1,2-a]pyrimidine |
HeLa (cervical) |
|
|
38 |
3-((4-methylpiperazin-1-yl)methyl)-2-phenylimidazo[1,2- α]pyrimidine |
HeLa (cervical) Panc-1 (pancreatic) MDA-MB-231 (breast) |
|
|
39 |
1,4-bis((2-(2-Tolyl)imidazo[1,2-a]pyrimidin-3-yl)methyl)piperazine |
HeLa (cervical) Panc-1 (pancreatic) |
|
|
40 |
3,5,7-trihydroxy-2-(4-hydroxyphenyl) -8-(piperidin-1-ylmethyl)-4H-chromen-4-one hydrochloride |
HeLa (cervical) HCC1954 (breast) SK-OV-3 (ovarian) |
|
|
41 |
2-(4-Hydroxy-(3-((4-(4-fluorophenyl) piperazin-1-yl)methyl) benzylidene) -2,3-dihydro-1H-inden-1-one |
HCA I and II (liver) |
|
|
42 |
4-(4-Chlorophenyl) -5-cyclohexyl-2-[(4-phenylpiperazin-1- yl)methyl]-2,4-dihydro-3H-1,2,4-triazole-3-thione |
T47D (breast) |
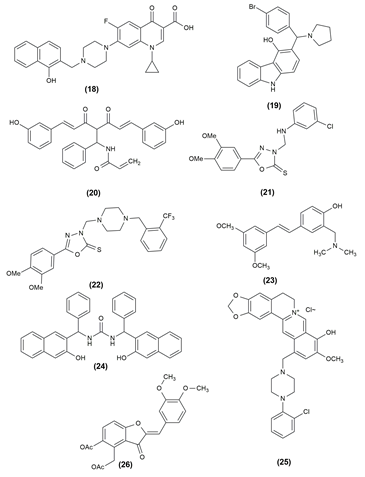
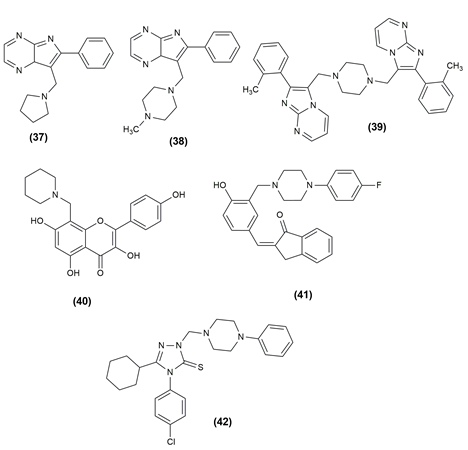
Alzheimer's Activity of Mannich bases
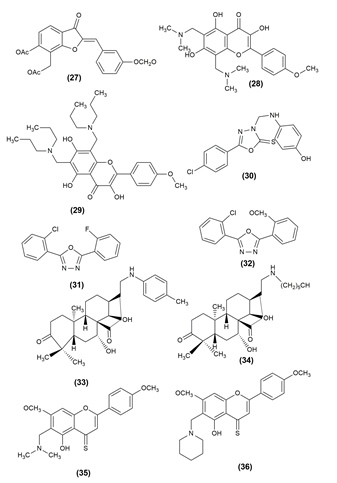
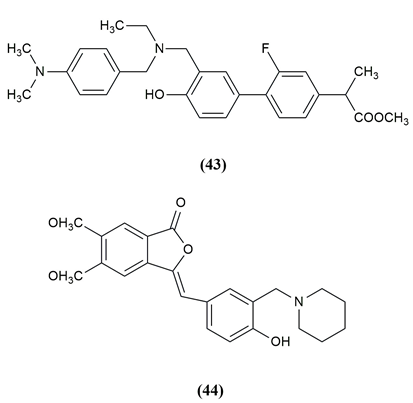
Cognitive decline and behavioural dysfunction are key features of Alzheimer's disease (AD), the most prevalent type of dementia in elderly people. Some of the mannich base compounds are given below which have strong inhibitory action against Alzheimer’s disease. 3-benzylidene/ benzylphthalide mannich base derivatives were evaluated as multifunctional agents and selective AChE, BuAChE and HuAChE inhibitors for the treatment of AD. The compound (43,44) Methyl 2 (-3’-((4-dimethylamino) benzyl)(ethyl)amino)methyl)-2-fluoro-4”hydroxyl-[1,1”-biphenyl]-4-yl) propanoate and (Z)-3-(4-hdroxy-3(piperidin-1-ylmethyl)benzylidene)-5,6-dimethoxyisobenzofuran-1(3H)-one exhibited good antiplatelet aggregation activity, Aβ1-42 aggregation inhibitor, biometal chelating ability anti-neuroinflammatory activity and appropriate BBB permeability and furthermore have a protective effect against H2O2-induced PC-12 cell damage, and they can ameliorate the scopolamine-induced learning and memory impairment.[7], [40] The compounds possessing anticholinesterase activity are given in [Figure 6].
Antidiabetic Activity of Mannich bases
A number of conditions, including genetic flaws, endocrinopathies, exocrine pancreatitis and infections, can result in diabetes, a metabolic illness. Type 2 diabetes mellitus (T2DM) makes up more than 90% of all cases among the different kinds. Structures containing a separate nabumetone moiety of β-amino ketone were obtained using a one-pot, two-step, three-component Mannich reaction protocol. The structure-activity relationship points to the sulphanilamide unit as the most likely potent group of b-amino ketone and on this basis, a material strategy for the development of new anti-diabetic drugs is presented. The compound (45), 5-(6-Methoxynaphthalen-2-yl)-1-(5-methylisoxazol-3-ylamino)-1-(3-nitrophenyl)pentan-3-one shown potent activity.[41]
Mannich bases of 1,3,4-oxadiazole derivatives incorporating benzothiazole scaffolds were synthesized and the antidiabetic activity was evaluated. In the STZ model, compound (46) 5-(Benzothiazol-2-yl) -3-[(3,4-dichlorophenylamino) methyl] 1, 3, 4 oxadiazol as well as 5-(Benzothiazol-2-yl)-3-[(2-methyl-4-nitrophenylamino)methyl] -1,3,4-oxadiazol-2(3H) -thione (47) showed the greatest blood glucose level reduction, on par with the conventional medication glibenclamide.[42] The compound possessing antidiabetic activity and the structures are given in [Figure 7] .
Anti-Inflammatory Activity of Mannich bases
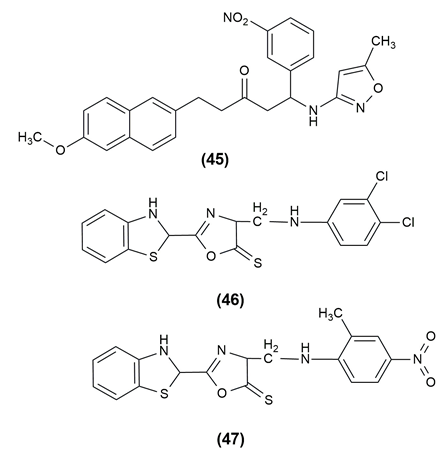
A widespread occurrence, inflammation is caused by the dynamic interplay of cellular and humoral responses through a number of inflammatory mediators. Cyclooxygenase (COX), the enzyme that controls the rate of the prostanoid biosynthesis pathway, catalyzes the transformation of arachidonic acid into critical inflammatory mediators such prostaglandins (PGs), prostacyclins and thromboxanes. Dehydrozingerone-based mannich bases were designed and evaluated for anti-inflammatory potential by using the inhibition of heat-induced albumin denaturation method. Among the various derivatives, the compound (48) was found to be having highly potent anti-inflammatory action.[43] (2E,6E)-2-({3-[(dimethylamino) methyl]-4-hydroxy-5-methoxyphenyl} methylidene)-6-(phenyl methylidene) cyclohexan-1-one (49) was synthesized and screened for anti-inflammatory activity via the inhibition of heat-induced albumin denaturation method. When compared to the standard drug diclofenac, the synthesized compound was found to be having higher anti-inflammatory potential.[44]
A new class of 5-(3,4-dichlorophenyl)-1,3,4-oxadiazole-2(3H)-one Mannich bases was synthesized and evaluated their potential as anti-inflammatory agents. To establish a non-toxic concentration for screening for anti-inflammatory effects, the target compounds were first evaluated for cytotoxicity. The anti-inflammatory activity of the compound (50), 5-(3,4-Dichlorophenyl)-3-[4-(piperidin-4-yl)piperidin-1-yl]-methyl-1,3,4-oxadiazol-2(3H)-one was better than that of the reference drug indomethacin.[45] The structures of the compounds possessing anti-inflammatory activity are given in [Figure 8].
Antitubercular Activity of Mannich Bases
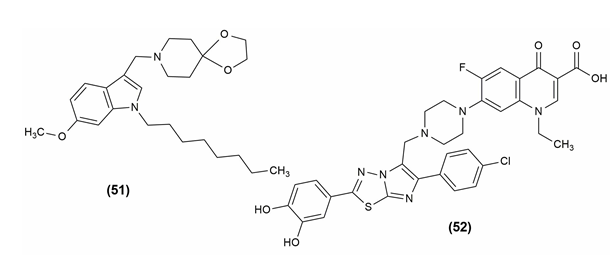
Despite advancements in our understanding of the infectious organism Mycobacterium tuberculosis, tuberculosis is still frustratingly challenging to treat and continues to claim a significant toll on human life. Malaria and HIV/AIDS are no longer the main infectious causes of death, respectively. Compound (51) (8-[(6-methoxy-1-octyl-1H-indol-3-yl)methyl]) -1,4-dioxa-8-azaspiro[4], [5]decane) is one of the potent derivatives of indolyl Mannich bases.[46]
The antimycobacterial capacity of the uniqueimidazo[2,1-b][1], [3], [4]thiadiazole (ITD) compounds was investigated. The 3,4-hydroxybenzene ring was fixed in the C-2 position of the ITD structure and the properties of the two series of compounds obtained by phenyl or 4-chlorophenyl in the C-6 position were compared. Mycobacterium smegmatis was found to be the most sensitive microorganism, with better MIC values in response to the standard drug Streptomycin. The compound 7-(4-{[2-(3,4-Dihydroxyphenyl)-6-(4-chlorophenyl) imidazo[2,1-b][1], [3], [4] thiadiazol-5-yl]methyl}piperazin-1-yl)-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolon-3-carboxylic acid (52) showed excellent anti-tubercular activity.[47] The structure of the compounds possessing anti-tubercular activity is given in [Figure 9].
Antioxidant Activity of Mannich Bases
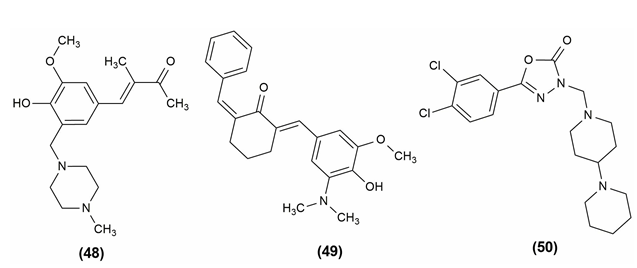
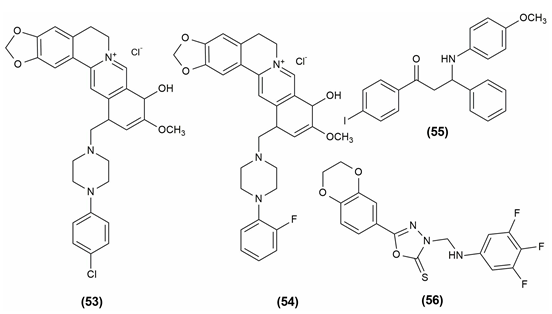
Antioxidants are substances that prevent oxidation, a chemical reaction that can result in the production of free radicals (typically seen as autoxidation). This might trigger further chain processes, like polymerization. As a result, substances with halogen functionality, 12-(1-(4-Chlorophenyl) piperazine-1-ylmethyl) -berberrubine (53) and 12-(1-(2-Fluorophenyl) piperazine-1-ylmethyl)-berberrubine (54) might be thought of as possessing a significant amount of antioxidant power in the relevant assay. According to functional group presence and activity relationships, the final analogs' antioxidant efficacies can be rated as follows: F > Cl > NO2 > CH3 > OCF3 > CF3. Based on Gibbs free energy, an in vitro DPPH radical experiment revealed the molecule containing the anisidine moiety, 1-(4-iodophenyl)-3-((4-methoxyphenyl)amino)-3-phenylpropan-1-one (55) has a superior radical scavenging activity.[5], [29]
The mannich base of 1,3,4-oxadiazole derivatives possessing 1,4-benzodioxan showed antioxidant activity. The most powerful activity was seen in compound (56), 5-(2,3-dihydrobenzo[b] 1,4 dioxin-6-yl)-3-(((3,4,5-trifluorophenyl)amino)methyl)-1,3,4- oxadiazole-2(3H)-thione i.e., having several fluoro substituents on the benzene.[48] The compound shows antioxidant activity and the structures are given in [Figure 10].
Antilipase Activity of Mannich bases
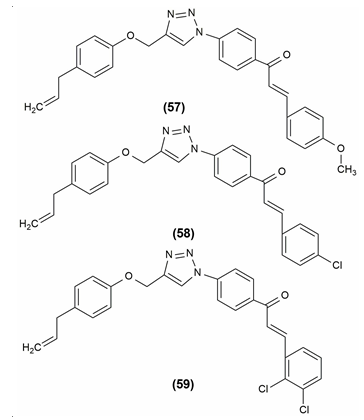
Inhibitors of lipases, which are present in the intestine, are called lipase inhibitors. When there is fat present, the pancreas secretes lipases. Lipase inhibitor’s main function is to lessen fat absorption from the gastrointestinal tract. When synthetic eugenol derivatives were tested in vitro for their ability to inhibit pancreatic lipase, compound (E)-1-(4-(4-([5-allyl-2-methoxyphenoxy]methyl)-1H1,2,3-triazol-1-yl)phenyl)-3-(4-methoxyphenyl)prop-2-en1-one (57) showed the most potent activity and the compounds (E)-1-(4-(4-([5-allyl-2-methoxyphenoxy]methyl)-1H1,2,3-triazol-1-yl)phenyl)-3-(4-chlorophenyl)prop-2-en1-one (58) and (E)-1-(4-(4-([5-allyl-2-methoxyphenoxy]methyl)-1H1,2,3-triazol-1-yl)phenyl)-3-(2,3-dichlorophenyl) prop-2-en-1-one (59) also exhibited moderate antilipase activity.[1] The structures of these compounds are given in [Figure 11].
Conclusion
In this review, the various biological applications of Mannich bases and their derivatives are discussed. The Mannich base and its derivatives, which are a significant class of molecules in synthetic chemistry, exhibit better pharmacological properties including antibacterial, anti-cancer, anti-inflammatory and antioxidant activities. Several biologically active, naturally occurring substrates or well-known medications have undergone chemical modification using the Mannich reaction in the last ten years to improve their biological activity. These mannich bases demonstrated better activities against various diseases with better potent action like the standard drugs. Thus, the Mannich reaction has established itself as a powerful tool in medicinal chemistry and can be used to synthesize novel chemical entities possessing a variety of exciting applications with better physicochemical properties.
Acknowledgment
We thank the Management and Dr. G. Murugananthan, Principal of our college for giving constant support and encouragement for writing this review.
Authors Contribution
All the authors have contributed equally.
Source of Funding
None
Disclosure of Conflict of Interest
The authors hereby disclose no conflicts of interest regarding the publication of this paper.
References
- Buduma K, Kumar N, Srinivas S, Kumar K, Chinde S, Domatti A. Synthesis and bioactivity evaluation of eugenol hybrids obtained by Mannich and 1,3 dipolar cycloaddition reactions. J Heterocyclic Chem. 2021;58(11):2078-89. [Google Scholar]
- Pishawikar S, More H. Synthesis, docking and in-vitro screening of mannich bases of thiosemicarbazide for anti-fungal activity. Arab J Chem. 2017;10(2):S2714-22. [Google Scholar]
- Abdel-Rahman I, Mustafa M, Mohamed S, Yahia R, AA, Abuo-Rahma M. Novel Mannich bases of ciprofloxacin with improved physicochemical properties, antibacterial, anticancer activities and caspase-3 mediated apoptosis. Bioorg Chem. 2021;107. [Google Scholar] [Crossref]
- Reddy P, Padmaja P, Reddy B, Jadav S. Synthesis in vitro antiproliferative activity, antioxidant activity and molecular modeling studies of new carbazole Mannich bases. Med Chem Res. 2017;26:2243-59. [Google Scholar]
- Petrović V, Simijonović D, Milovanović V, Petrović Z. Acetophenone Mannich bases: study of ionic liquid catalysed synthesis and antioxidative potential of products. R Soc Open Sci. 2018;5(11). [Google Scholar] [Crossref]
- Rajasekharan S, Kamalanathan C, Ravichandran V, Ray A, Satish A, Mohanvel S. Mannich base limits Candida albicans virulence by inactivating Ras-cAMP-PKA pathway. Sci Rep. 2018;8(1). [Google Scholar]
- Cao Z, Song Q, Yu G, Liu Z, Cong S, Tan Z. Novel 3-benzylidene/benzylphthalideMannich base derivatives as potential multifunctional agents for the treatment of Alzheimer's disease. Bioorg Med Chem. 2021;35. [Google Scholar] [Crossref]
- Fu Y, Yang Y, Zhou S, Liu Y, Yuan Y, Li S. Ciprofloxacin containing Mannich base and its copper complex induce antitumor activity via different mechanism of action. Int J Oncol. 2014;45(5):2092-100. [Google Scholar]
- Pape V, Palkó R, Tóth S, Szabó MJ, Sessler J, Dormán G. Structure-Activity Relationships of 8-Hydroxyquinoline-Derived Mannich Bases with Tertiary Amines Targeting Multidrug-Resistant Cancer. J Med Chem. 2022;65(11):7729-45. [Google Scholar]
- Lamberth C. Multicomponent reactions in crop protection chemistry. Bioorg Med Chem. 2020;28(10). [Google Scholar] [Crossref]
- Zhang Y, Han J, Liu Z. Diaryliodonium salts as efficient lewis acid catalyst for direct three component Mannich reactions. RSC Adv. 2015;5:25485-8. [Google Scholar]
- Singh M. Synthesis, Characterization and physiochemical investigsation of Cu(II) Complexes with Newly Prepared Mannich Base Ligands Derived from Hetrocycles with Sulphacetamide/Sulphanilamide. Acta Ciencia Indica. 2017;43(4). [Google Scholar]
- Hala M, Alzahawi G. Synthesis, characterization and antimicrobial activity of shiff and mannich bases of uracil derivatives. Kirkuk University J Sci Stud. 2016;11(1):124-54. [Google Scholar]
- Al-Tuwaijri AE, Hassan H, Haiba H, Habib M, El-Emam E. Antimicrobial and hypoglycemic activities of novel N-Mannich bases derived from 5-(1-adamantyl)-4-substituted-1,2,4-triazoline-3-thiones. Int J Mol Sci. 2014;15(12):22995-3010. [Google Scholar]
- Hatamleh A, DAF, Al-Saif S, Chidambaram S, Radhakrishnan S. Cytotoxic Analysis, and Molecular Docking Studies of Tetrazole Derivatives via N-Mannich Base Condensation as Potential Antimicrobials. Drug Des Devel Ther. 2020;14:4477-92. [Google Scholar]
- Zhang Y, Liu X, Zhan Y, Zhang L, Li Z, Li Y. Synthesis and biological activities of novel 5-substituted-1,3,4-oxadiazole Mannich bases and bis-Mannich bases as ketol-acid reductoisomerase inhibitors. Bioorg Med Chem Lett. 2016;26(19):4661-5. [Google Scholar]
- Abrão P, Pizi R, Souza TD, Silva N, Fregnan A, Silva F. Synthesis and Biological Evaluation of New Eugenol Mannich Bases as Promising Antifungal Agents. Chem Biol Drug Des. 2015;86(4):459-65. [Google Scholar]
- Lv XH, Liu H, Ren ZL, Wang W, Tang F, Cao H. Design, synthesis and biological evaluation of novel flavone Mannich base derivatives as potential antibacterial agents. Mol Divers. 2019;23(2):299-306. [Google Scholar]
- Yang C, Li T, Jiang L, Zhi X, Cao H. Semisynthesis and biological evaluation of some novel Mannich base derivatives derived from a natural lignan obovatol as potential antifungal agents. Bioorg Chem. 2019;94. [Google Scholar]
- Ünver Y, Deniz S, Çelik F, Akar Z, Küçük M, Sancak K. Synthesis of new 1,2,4-triazole compounds containing Schiff and Mannich bases (morpholine) with antioxidant and antimicrobial activities. J Enzyme Inhib Med Chem. 2016;31(3):89-95. [Google Scholar]
- Triloknadh S, Rao V, Nagaraju C, Ramaiah V, Krishna H, Suneetha D. Design, synthesis, neuroprotective, antibacterial activities and docking studies of novel thieno[2,3-d]pyrimidine-alkyne Mannich base and oxadiazole hybrids. Bioorg Med Chem Lett. 2018;28(9):1663-9. [Google Scholar]
- Świątek P, Strzelecka M. IsothiazolopyridineMannich bases and their antibacterial effect. Adv Clin Exp Med. 2019;28(7):967-72. [Google Scholar]
- Shi J, Ding M, Luo N, Wan S, Li P, Li J. Design, Synthesis, Crystal Structure, and Antimicrobial Evaluation of 6-Fluoroquinazolinylpiperidinyl-Containing 1,2,4-Triazole Mannich Base Derivatives against Phytopathogenic Bacteria and Fungi. J Agric Food Chem. 2020;68(36):9613-23. [Google Scholar]
- Sethi R, Jain S, Arora S, Saini D, Jain N, Synthesis. Characterization and Molecular Docking Studies of Novel N-(benzimidazol-1-ylmethyl)-4-chlorobenzamide Analogues for Potential Anti-inflammatory and Antimicrobial Activity. Antiinflamm Antiallergy Agents Med Chem. 2018;17(1):16-31. [Google Scholar]
- Gyuris M, Hackler L, Nagy LI, Alföldi R, Rédei E, Marton A. Mannich Curcuminoids as Potent Anticancer Agents. Arch Pharm. 2017. [Google Scholar] [Crossref]
- Al-Wahaibi L, Mohamed A, Tawfik S, Hassan H, El-Emam A. 1,3,4-Oxadiazole N-Mannich Bases: Synthesis, Antimicrobial, and Anti-Proliferative Activities. Molecules. 2021;1. [Google Scholar] [Crossref]
- Nguyen V, Shi L, Luan F, Wang Q. Synthesis of kaempferide Mannich base derivatives and their antiproliferative activity on three human cancer cell lines. Acta Biochim Pol. 2015;62(3):547-52. [Google Scholar]
- Bajaj S, Roy P, Singh J. Synthesis, thymidine phosphorylase inhibitory and computational study of novel 1,3,4-oxadiazole-2-thione derivatives as potential anticancer agents. Comput Biol Chem. 2018;76:151-60. [Google Scholar] [Crossref]
- Bajaj S, Kumar M, Tinwala H, Yc M. Design, synthesis, modelling studies and biological evaluation of 1,3,4-oxadiazole derivatives as potent anticancer agents targeting thymidine phosphorylase enzyme. Bioorg Chem. 2021;111. [Google Scholar] [Crossref]
- Yang J, Liu Y, Xue C, Yu W, Zhang J, Qiao C. Synthesis and biological evaluation of glaucocalyxin A derivatives as potential anticancer agents. Eur J Med Chem. 2014;86:235-76. [Google Scholar] [Crossref]
- Li W, Li X, Liu M, Wang Q. Synthesis and Antiproliferative Activity of ThioxoflavonesMannich Base Derivatives. Arch Pharm (Weinheim). 2017. [Google Scholar] [Crossref]
- Aeluri R, Polepalli A, Jain S. Synthesis and antiproliferative activity of imidazo[1,2-a]pyrimidineMannich bases. Eur J Med Chem. 2015;100:18-23. [Google Scholar]
- Cai S, Kong Y, Chen X, Wang Y. Primary aminomethyl derivatives of kaempferol: hydrogen bond-assisted synthesis, anticancer activity and spectral properties. Org Biomol Chem. 2018;16(11):1921-31. [Google Scholar]
- Popiołek �, Rzymowska J, Kosikowska U, Hordyjewska A, Wujec M, Malm A. Synthesis, antiproliferative and antimicrobial activity of new Mannich bases bearing 1,2,4-triazole moiety. J Enzyme Inhib Med Chem. 2014;29(6):786-95. [Google Scholar]
- Liu C, Dong L, Wang S, Wang Q. Synthesis and antiproliferative activity of pterostilbene and 3′-methoxy pterostilbene Mannich base derivatives against Hela cells. Mol Divers. 2015;19(4):737-80. [Google Scholar]
- Vedarethinam V, Dhanaraj K, Ilavenil S, Arasu M, Choi K, Al-Dhabi N. -Hydroxynaphthalen-2-yl)phenylmethyl)urea) on Hepatocellular Carcinoma. Indian J Clin Biochemis. 2016;21(1). [Google Scholar]
- Mistry B, Patel R, Keum Y, Kim D. Synthesis of N-Mannich bases of berberine linking piperazine moieties revealing anticancer and antioxidant effects. Saudi J Biol Sci. 2017;24(1):36-44. [Google Scholar]
- Popova A, Frasinyuk M, Bondarenko S, Zhang W, Xie Y, Martin Z. Efficient Synthesis of Aurone Mannich Bases and Evaluation of their Antineoplastic Activity in PC-3 Prostate Cancer Cells. Chem Zvesti. 2018;72(10):2443-56. [Google Scholar]
- Tugrak M, Gul H, Bandow K, Sakagami H, Gulcin I, Ozkay Y. Synthesis and biological evaluation of some new mono Mannich bases with piperazines as possible anticancer agents and carbonic anhydrase inhibitors. Bioorg Chem. 2019;90. [Google Scholar] [Crossref]
- Liu H, Qiang X, Song Q, Li W, He Y, Ye C. Discovery of 4'-OH-flurbiprofen Mannich base derivatives as potential Alzheimer's disease treatment with multiple inhibitory activities. Bioorg Med Chem. 2019;27(6):991-1001. [Google Scholar]
- Wang H, Yan J, Song X, Fan L, JXu, Zhou G. Synthesis and antidiabetic performance of β-amino ketone containing nabumetone moiety. Bioorg Med Chem. 2012;20(6):2119-49. [Google Scholar]
- Bhutani R, Pathak D, Kapoor G, Husain A, Kant R, Iqbal M. Synthesis, molecular modelling studies and ADME prediction of benzothiazole clubbed oxadiazole-Mannich bases, and evaluation of their anti-diabetic activity through in vivo model. Bioorg Chem. 2018;77:6-15. [Google Scholar]
- Hayun H, Arrahman A, Purwati E, Yanuar A, Fortunata F, Suhargo F. Synthesis, Anti-inflammatory, and Antioxidant Activity of Mannich Bases of Dehydrozingerone Derivatives. J Young Pharm. 2018;10(2):6-10. [Google Scholar]
- Hayun H, Maggadani B, Kurnia A, Hanifah A, Yuliandi M, Fitriyani I. Anti-Inflammatory and Antioxidant Activity of Synthesized Mannich Base Derivatives of (2e,6e)-2-[(4-Hydroxy-3-Methoxyphenyl)Methylidene]-6-(Phenyl Methylidene)Cyclohexan-1-One. Int J App Pharm. 2019;11(1):246-50. [Google Scholar]
- Koksal M, Dagliyan I, Ozyazici T, Kadioglu B, Sipahi H, Bozkurt A. Some Novel Mannich Bases of 5-(3,4-Dichlorophenyl)-1,3,4-oxadiazole-2(3H)-one and Their Anti-Inflammatory Activity. Arch Pharm. 2017. [Google Scholar] [Crossref]
- Nyantakyi S, Li M, Gopal P, Zimmerman M, Dartois V, Gengenbacher M. Indolyl AzaspiroketalMannich Bases Are Potent Antimycobacterial Agents with Selective Membrane Permeabilizing Effects and in Vivo Activity. J Med Chem. 2018;61(13):5733-50. [Google Scholar]
- Taflan E, Bayrak H, Er M, Alpaykaraoğlu �, Bozdeveci A. (ITD) hybrid compounds: Design, synthesis, efficient antibacterial activity and antioxidant effects. Bioorg Chem. 2019;89. [Google Scholar] [Crossref]
- Ma L, Xiao Y, Li C, Xie Z, Li D, Wang Y. Synthesis and antioxidant activity of novel Mannich base of 1,3,4-oxadiazole derivatives possessing 1,4-benzodioxan. Bioorg Med Chem. 2013;21(21):6763-70. [Google Scholar]
- Abstract
- Introduction
- Antimicrobial Activity of Mannich bases
- Anticancer Activity of Mannich bases
- Alzheimer's Activity of Mannich bases
- Antidiabetic Activity of Mannich bases
- Anti-Inflammatory Activity of Mannich bases
- Antitubercular Activity of Mannich Bases
- Antioxidant Activity of Mannich Bases
- Antilipase Activity of Mannich bases
- Conclusion
- Acknowledgment
- Authors Contribution
- Source of Funding
- Disclosure of Conflict of Interest
- References
How to Cite This Article
Vancouver
Raju SK, Vengadhajalaphathy P, Sundaram R, Periyasamy S, Chinnaraj T, Sekar P. Recent advances in biological applications of mannich bases — An overview [Internet]. Int J Pharm Chem Anal. 2023 [cited 2025 Nov 03];10(1):15-27. Available from: https://doi.org/10.18231/j.ijpca.2023.004
APA
Raju, S. K., Vengadhajalaphathy, P., Sundaram, R., Periyasamy, S., Chinnaraj, T., Sekar, P. (2023). Recent advances in biological applications of mannich bases — An overview. Int J Pharm Chem Anal, 10(1), 15-27. https://doi.org/10.18231/j.ijpca.2023.004
MLA
Raju, Senthil Kumar, Vengadhajalaphathy, Priyadharshini, Sundaram, Revathy, Periyasamy, Sangeetha, Chinnaraj, Thatchayani, Sekar, Praveen. "Recent advances in biological applications of mannich bases — An overview." Int J Pharm Chem Anal, vol. 10, no. 1, 2023, pp. 15-27. https://doi.org/10.18231/j.ijpca.2023.004
Chicago
Raju, S. K., Vengadhajalaphathy, P., Sundaram, R., Periyasamy, S., Chinnaraj, T., Sekar, P.. "Recent advances in biological applications of mannich bases — An overview." Int J Pharm Chem Anal 10, no. 1 (2023): 15-27. https://doi.org/10.18231/j.ijpca.2023.004
